Talking about aging, lysosomes have something to say
For decades, biology students have learned that lysosomes – tiny sacs found within nearly all cells – had a singular task: to gobble up bits of foreign material and worn-out cell parts. The aim? To break them down for recycling. But that lesson might soon need revising.
Dr. Meng Wang, professor and Robert C. Fyfe Endowed Chair on Aging of Molecular and Human Genetics and an investigator at HHMI at Baylor, has spent the past seven years exploring the link between longevity and the signals lysosomes produce.

Longevity, it seems, runs deep in Wang’s blood – her paternal and maternal grandmothers lived until ages 100 and 95, respectively. “Neither was ill, and why they died remains a mystery,” she said. “It makes me feel like there’s some secret to longevity, and if we can study this, maybe we can use the knowledge and apply it to everyone.”
“Cells in different organs and tissues around the body send signals to one another constantly,” said Wang, a member of Baylor’s Dan L Duncan Comprehensive Cancer Center. “When we are young, everything is connected and communicating, but as we age, some of these connections are lost and function declines.”
Extending life
By 2050, scientists expect the number of people worldwide age 65 years and up to double to 1.5 billion, according to a 2019 report from the United Nations. And growing older, scientists know, comes with an increased likelihood of disease and other health problems.
“That explains why research on lysosomes and longevity has taken off in recent years,” said Dr. Xiaochen Wang, a biophysicist at the Chinese Academy of Sciences (CAS) in Beijing who specializes in lysosomes. “Scientists have known for more than 50 years that proteins, lipids and other molecules tend to accumulate in cells that are old, but only now are people paying more attention to these pieces of data,” Xiaochen Wang said.
Because lysosomes are the dumping site for cellular waste products, researchers are studying them in greater detail, suspecting a connection to aging. Within the past decade, scientists have shown that lysosomes serve as a platform the cell uses to send messages about its general state, such as its glucose and stress levels.
In 2015, Meng Wang’s group was one of the first to discover specific anti-aging signals produced by lysosomes in the tiny roundworm C. elegans.
Xiaochen Wang says the work stands out because it was conducted in a living organism, rather than in cultured cells researchers typically use to study lysosomes. Additionally, those experiments showed how one particular molecule released from lysosomes traveled to the cell’s nucleus and acted as a signal that extended the worm’s lifespan. “It’s a very beautiful piece of work,” she said.
From one cell to another
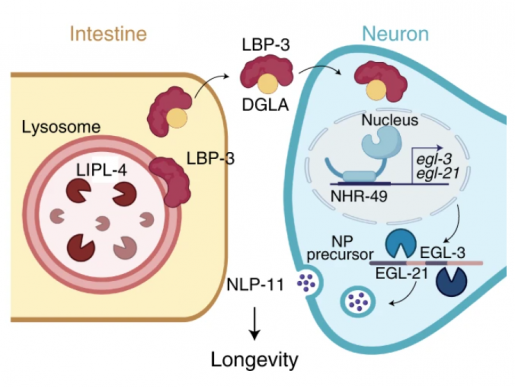
This study takes the research one step further. In work led by Meng Wang’s Ph.D. student Marzia Savini, the Baylor team discovered that when lysosomes produce a fatty acid called dihomo-gamma-linoleic acid, a chain reaction of cellular messages begins and ultimately extends the worms’ lives. When the researchers dialed up this fatty acid signaling, worms lived 20 to 25 days, an increase from the normal lifespan of 17 days. Crucially, the signal molecule was generated in fat tissues, yet detected by neurons elsewhere in the worms.
Lysosomes produce signals that cells use to coordinate longevity across different tissues.
“It really helps us understand how longevity is regulated at a whole organism level,” Meng Wang said.
Today, Meng Wang and her team continue to hunt for other molecules lysosomes produce that may act as anti-aging signals. “There’s still so much to uncover,” she said, “such as figuring out when the molecules begin acting as signals, how they regulate aging and how that function withers with age.”
The goal is not to have everyone live for centuries,” Meng Wang said, “but rather for people to spend their last years healthy, active and independent – just like my grandmothers. I’m passionate to know how people and organisms sustain health with time.”
Find all the details of this study in the journal Nature Cell Biology.
Andrew Folick, Yi-Tang Lee, Feng Jin, André Cuevas, Matthew C. Tillman, Jonathon D. Duffy, Qian Zhao, Isaiah A. Neve, Pei-Wen Hu, Yong Yu, Qinghao Zhang, Youqiong Ye, William B. Mair, Jin Wang, Leng Han and Eric A. Ortlund also contributed to this work. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine, Howard Hughes Medical Institute at Baylor, University of Texas Health Science Center at Houston, University of California San Francisco, Emory University School of Medicine, Harvard T.H. Chan School of Public Health Boston and Texas A&M University, Houston.
This work was supported by NIH grants R01AG045183, R01AT009050, R01AG062257, DP1DK113644, P01AG066606, RF1AG074540, T32 ES027801 pre-doctoral fellowships R03AG070417, R01HG011633 and R01CA262623, Welch Foundation Q-1912-20190330, and HHMI investigator support.



