Engineering meets biology to design innovative multifunctional surgical Biomesh
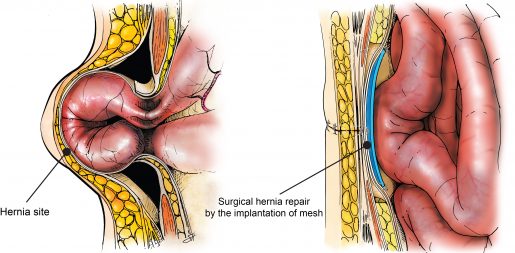
Hernias form when intra-abdominal content, such as a loop of the intestine, squeezes through weak, defective or injured areas of the abdominal wall.
The condition, one of the most common soft tissue injuries, may develop serious complications, therefore hernia repair may be recommended. Repair consists of surgically implanting a prosthetic mesh to support and reinforce the damaged abdominal wall and facilitate the healing process. However, currently used mesh implants are associated with potentially adverse postsurgical complications.
“Although hernia mesh implants are mechanically strong and support abdominal tissue, making the patient feel comfortable initially, it is a common problem that about three days after surgery the implant can drive inflammation that in two to three weeks will affect organs nearby,” said Dr. Crystal Shin, assistant professor of surgery at Baylor College of Medicine and lead author of this study looking to find a solution to postsurgical hernia complications.

Mesh implants mostly fail because they promote the adhesion of the intestine, liver or other visceral organs to the mesh. As the adhesions grow, the mesh shrinks and hardens, potentially leading to chronic pain, bowel obstruction, bleeding and poor quality of life. Some patients may require a second surgery to repair the unsuccessful first.
“Inflammation is also a serious concern,” said Dr. Ghanashyam Acharya, associate professor of surgery at Baylor. “Currently, inflammation is controlled with medication or anti-inflammatory drugs, but these drugs also disturb the healing process because they block the migration of immune cells to the injury site.”
“To address these complications, we developed a non-pharmacological approach by designing a novel mesh that, in addition to providing mechanical support to the injury site, also acts as an inflammation modulating system,” Shin said.
Opposites attract
“A major innovation to our design is the development of a Biomesh that can reduce inflammation and, as a result, minimize tissue adhesion to the mesh that leads to pain and failure of the surgery,” Shin said.
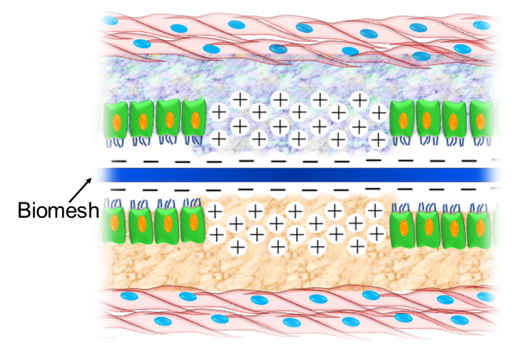
Inflammatory mediators called cytokines appear where the mesh is implanted a few days after the surgery. Some of the main cytokines in the implant, IL1-β, IL6 and TNF-α, have a positive surface charge due to the presence of the amino acids lysine and arginine.

“We hypothesized that Biomesh with a negative surface charge would capture the positively charged cytokines, as opposite electrical charges are attracted to each other,” Acharya said. “We expected that trapping the cytokines in the mesh would reduce their inflammatory effect and improve hernia repair and the healing process.”
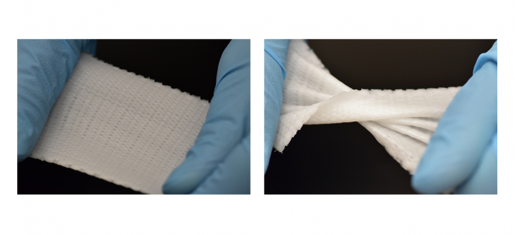
To test their new idea, the researchers used a 3-D-bioprinter to fabricate Biomesh of a polymer called phosphate crosslinked poly (vinyl alcohol) polymer (X-PVA). Through thorough experimentation, they optimized the mechanical properties so the mesh would withstand maximal abdominal pressure repeatedly without any deterioration of its mechanical strength for several months. They also showed that their Biomesh did not degrade or reduce its elastic properties over time and was not toxic to human cells.
Shin, Acharya and their colleagues have confirmed in the lab that this Biomesh can capture positively charged cytokines.
Encouraged by these results, the researchers tested their Biomesh in a rat model of hernia repair, comparing it with a type of mesh extensively used clinically for surgical hernia repair.
Newly designed 3-D printed Biomesh minimizes postsurgical complications of hernia repair in an animal model
The newly designed Biomesh effectively minimized postsurgical complications of hernia repair in an animal model. The researchers examined the Biomesh for four weeks after it was implanted. They found that the newly designed Biomesh had captured about three times the amount of cytokines captured by the commonly used mesh. Cytokines are short-lived in the body. As they degrade, they enable the mesh to capture more cytokines.
Importantly, no visceral tissues had adhered to the newly designed Biomesh, while the level of tissue adhesion was extreme in the case of the commonly used mesh. These results confirmed that the new Biomesh is effective at reducing the effects of the inflammatory response and in preventing visceral adhesions. In addition, the new mesh did not hinder abdominal wall healing after surgical hernia repair in animal models.
“This Biomesh is unique and designed to improve outcomes and reduce acute and long-term complications and symptoms associated with hernia repair.”
With more than 400,000 hernia repair surgeries conducted every year in the U.S., the new Biomesh would fulfill a major unmet need,” Shin said.
“There is no such multifunctional composite surgical mesh available, and development of a broadly applicable Biomesh would be a major advancement in the surgical repair of hernia and other soft tissue defects. We are conducting further preclinical studies before our approach can be translated to the clinic. Fabricating the Biomesh is highly reproducible, scalable and modifiable.”
“This concept of controlling inflammation through the physicochemical properties of the materials is new. The mesh was originally designed for mechanical strength. We asked ourselves, can we create a new kind of mesh by making use of the physical and chemical properties of materials?” said Acharya. “In the 1950s, Dr. Francis C. Usher at Baylor’s Department of Surgery developed the first polypropylene mesh for hernia repair.
We have developed a next-generation mesh that not only provides mechanical support but also plays a physiological role of reducing the inflammatory response that causes significant clinical problems.”
Read the complete study in the journal Advanced Materials.
Other contributors to this work include Fernando J. Cabrera, Richard Lee, John Kim, Remya Ammassam Veettil, Mahira Zaheer, Kirti Mhatre and Bradford G. Scott who are affiliated with Baylor College of Medicine. Aparna Adumbumkulath and Pulickel M. Ajayan are at Rice University and Steven A. Curley is at Christus Health Institute.
This work was supported by Baylor College of Medicine seed funding.
Learn more about the Shin lab and the Acharya lab:
Current research interests of the Shin lab focus on developing broadly applicable drug delivery systems for surgical applications with enhanced therapeutic efficacy by integrating nanotechnology and 3-D bioprinting technology. She is currently working on developing controlled release nanowafer therapeutics (a hydrogel-based drug delivery system), nanodrug delivery systems for wound healing and pain management, and theranostics, a combination of therapeutics and diagnostics, for image-guided drug delivery.
Acharya’s research program focuses on the development of advanced materials for regenerative engineering by integrating nanofabrication, 3-D-nanolithography and controlled drug delivery strategies. He works at the interface of medicine, bioengineering, chemistry and pharmaceutics.