Connecting Zika virus and hereditary microcephaly
Understanding how Zika virus causes microcephaly would hint at possibilities for preventing this irreparable condition in newborns. Heading in that direction, a collaboration between Baylor College of Medicine and the University of California, San Francisco has revealed interesting insights into the interactions between Zika virus proteins and host proteins, including human proteins.
“In this study, we collaborated with Dr. Nevan J. Krogan and Dr. Priya Shah at the University of California, San Francisco to better understand the mechanisms of dengue and Zika virus infection,” said Dr. Hugo Bellen, professor of molecular and human genetics and neuroscience at Baylor College of Medicine and an investigator at the Howard Hughes Medical Institute.

The researchers conducted systematic comparative analyses of the interactions of proteins from dengue and Zika viruses with proteins from the host, both mosquitoes and humans. They discovered new strategies the viruses use to successfully infect their host. For instance, they found that some viral proteins counteract interferon response genes, a human and mosquito defense mechanism, and that other viral proteins hijack host proteins and redirect their activities to replicate the virus.
In addition, the researchers combined their systematic comparative analysis with experiments with the fruit fly animal model and discovered an intriguing mechanism that can explain infant microcephaly associated with maternal Zika virus infection.
The Bellen lab combines the versatility of the fruit fly with modern molecular biology techniques to answer important questions about genes and disease. The technology has allowed scientists to determine the role of a gene and the corresponding protein in cells where the gene is expressed and whether the loss of the gene may be associated with human disease.
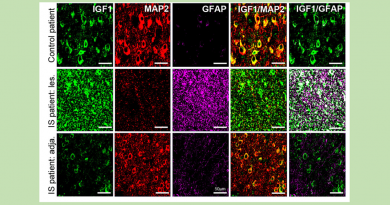
“We have altered thousands of flies and systematically characterize the expression and function of genes in great detail. In a previous study, we combined this approach with analyses of human genes not yet linked to human disease. We found that mutations in the ANKLE2 gene can cause microcephaly both in humans and fruit flies,” said Bellen, who also is a member of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital.
Microcephaly caused by lack of ANKLE2 is very similar to the one caused by Zika virus,” Bellen said.
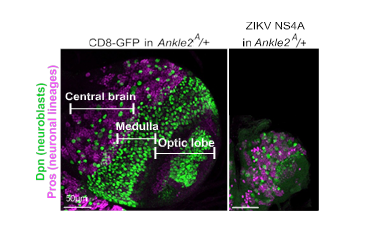
In this study, the researchers discovered that the Zika protein NS4A binds to ANKLE2, the human protein linked to microcephaly.

“We found that if we overexpress NS4A in normal flies, the result is flies with smaller brains. This can be rescued by overexpressing human ANKLE2 in the flies,” said co-first author, Dr. Nichole Link a postdoctoral associate in the Bellen lab.
Taken together, the evidence suggests that when the Zika virus protein NS4A interacts with ANKLE2, it disrupts its function in brain development in ways that can lead to microcephaly. These findings also suggest that, if we developed a drug that could prevent NS4A from binding to ANKLE2, we might be able to prevent Zika virus from causing microcephaly,” Link said.
Find all the details of this study in the journal Cell.
Other contributors to this work include Priya S. Shah (co-first author), Gwendolyn M. Jang, Phillip P. Sharp, Tongtong Zhu, Danielle L. Swaney, Jeffrey R. Johnson, John Von Dollen, Holly R. Ramage, Laura Satkamp, Billy Newton, Ruth Hüttenhain, Marine J. Petit, Tierney Baum, Amanda Everitt, Orly Laufman, Michel Tassetto, Michael Shales, Erica Stevenson, Gabriel N. Iglesias, Leila Shokat, Shashank Tripathi, Vinod Balasubramaniam, Laurence G. Webb, Sebastian Aguirre, A. Jeremy Willsey, Adolfo García-Sastre, Katherine S. Pollard, Sara Cherry, Andrea V. Gamarnik, Ivan Marazzi, Jack Taunton, and Ana Fernández-Sesma. For a complete list of the authors’ affiliations visit this link.
This work was supported by NIH/NIAID F32AI112262, NIH/NINDS F32NS092270, DOD/DARPA HR0011-11-C-0094 (PROPHECY), NIH/NIAID U19AI1186101, NIH/NIGMS P50 GM082250, NIH/NIAID R01AI07345, R21AI116022. Further support was provided by NIH U54NS093793, NIH R24OD022005, the Huffington Foundation and the Howard Hughes Medical Institute.