There are new key players in Parkinson’s disease
The roadmap that may lead to Parkinson’s disease has a number of ‘landmarks,’ gene variants and cellular defects known to be associated with the disease. Nevertheless, says Dr. Hugo Bellen, there is still little understanding of how these genes and pathways cause this condition.
“In this work, we have identified new contributors to the disease that we propose can provide a connection between previously unconnected genes and pathways observed in this disease,” said Bellen, professor of molecular and human genetics and neuroscience at Baylor College of Medicine and an investigator at the Howard Hughes Medical Institute.

Interestingly, when Dr. Guang Lin, postdoctoral associate in molecular and human genetics at Baylor, began working on this project in the Bellen lab, he was not looking to connect ‘landmarks’ in the roadmap to Parkinson’s disease.
“When I joined the laboratory of Dr. Bellen, I decided to work on the human PLA2GA6 gene. Mutations in this gene cause neurodegenerative disorders, including Parkinsonism,” said Lin. “The PLA2G6 gene encodes a phospholipase, an enzyme that modifies a type of fats called phospholipids. Phospholipids are major building blocks of our nervous system, but they have not been well characterized. We thought that we ought to investigate what this phospholipase was doing in these diseases. What we found was totally unexpected.”
Lin and his colleagues developed a fruit fly model of the human condition by knocking out the fly equivalent of the human PLA2GA6 gene, called iPLA2-VIA, which the researchers determined is expressed in neurons and probably other types of cells. These mutant fruit flies lived about one third of the lifespan of normal flies and presented with cellular characteristics similar to those observed in humans with mutations in PLA2G6 gene.
“Confirming previous results by other researchers, we also observed that fruit flies without the iPLA2-VIA gene were healthy when they were young and presented with age-dependent neurodegeneration,” Lin said.

In addition, the researchers looked for the first time at the effects of the mutation on two other measurements: bang sensitivity and the workings of the visual system. Bang sensitivity refers to the response of the fruit flies to mechanical stress. They observed that while normal fruit flies have no bang sensitivity, meaning they recover immediately from impact, flies lacking iPLA2-VIA gene take longer to recover; they were paralyzed for some time after the impact, indicating problems with their nervous system.
To explore the workings of the visual system, the researchers conducted electroretinograms to measure the electrical responses of photoreceptors at different times during the life of adult fruit flies. They found progressive loss of electrical activity in the mutant flies, findings that once again showed a defect in the nervous system of these flies.
“We further carried out electron microscopy studies to take a closer look at the structure of neurons. We discovered that in the sick flies, the photoreceptors had many inclusions, lump-like structures, in their membranes, which are a symptom that something is wrong. The inclusions were absent in normal cells,” said Bellen, who also is a member of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital.
“We also found structural malformations in most of the mitochondria, which is often a feature of Parkinson’s disease, as well as dramatic enlargement of lysosomes, intracellular structures involved in the recycling of membranes and other cellular components,” said Lin. “Altogether, these results indicated that the iPLA2-VIA gene is important to maintain proper membrane structure and shape.”
A surprising finding
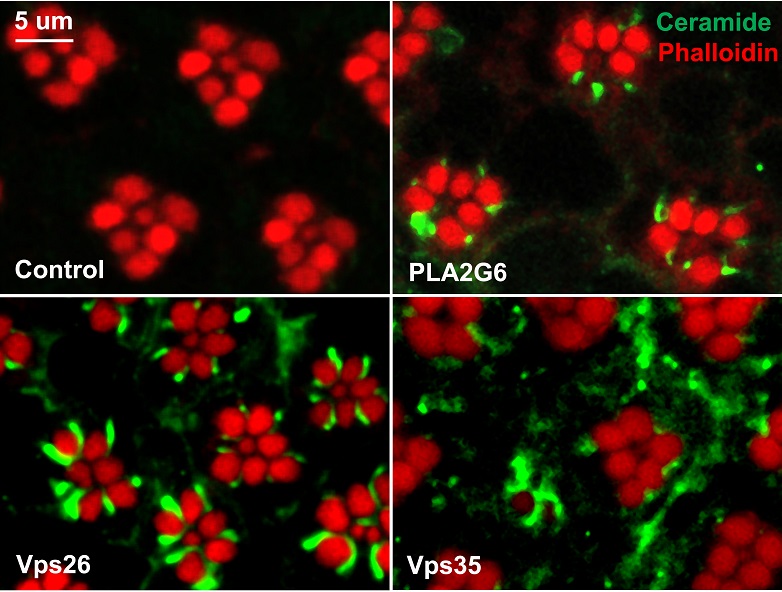
“Because the fly iPLA2-VIA gene encodes a phospholipase, an enzyme that modifies phospholipids, we expected that flies lacking the iPLA2-VIA gene would have changes in the phospholipids, providing a connection with the structural and functional defects we observed in the flies,” Bellen said. “Surprisingly, our analysis showed that the phospholipids were normal. We did not anticipate this.”
Then, what was abnormal?
The researchers looked at many types of lipids and found that the amounts of almost all the ceramides – lipids that are important for membrane structure and function – were increased in flies lacking the iPLA2-VIA gene. This suggested that the gene plays a role in the pathway that produces ceramides.
“We tested the effect of desipramine and myriocin, two drugs that block ceramide synthesis, on fruit flies lacking the iPLA2-VIA gene,” Bellen said. “As expected, both drugs led to decreased amounts of ceramides in the cells.”
Interestingly, mutant flies treated with the drugs also presented with less neurodegeneration, less bang sensitivity, improved electroretinograms and less alterations in the lysosomes when compared with mutant flies not treated with the drugs,” Bellen said.
Digging deeper into these results, the researchers looked into the processes cells use to reuse and to recycle ceramide-derived lipids in cell membranes. Cells reuse these lipids via intracellular trafficking mediated by the retromer. Retromers can identify these lipids, extract them before they are recycled and bring them back to membranes. Lipids that are not reused in this way are transported to the lysosomes where they are broken down into ceramides, which are then incorporated into membranes of the cells.
The increased levels of ceramide stiffen the membranes. This further disrupts the function of the retromer, leading to more lipid shuttling to the lysosomes where they are broken down to produce more ceramides. This creates a positive-feedback loop that causes ceramides to accumulate and leads to neurodegeneration.
The results of their investigations revealed clues suggesting that ceramide balance is disrupted in flies lacking the iPLA2-VIA gene. These mutant flies have decreased levels of Vps35 and Vps26, proteins in retromers that are essential for their function. The researchers found that normally the iPLA2-VIA protein binds to Vps35 and Vps26 and this binding enhances retromer function. Lacking iPLA2-VIA protein results in less Vps35 and Vps26 and causes the disruption of retromer function, suggesting that the iPLA2-VIA protein stabilizes retromers and their function, which was not known before.
“These observations led us to predict that if retromers are defective, then lysosomes would have extra work and should expand, and this is what we observed,” Bellen said. “All the approaches we tested that improved retromer function also resulted in an improvement of the defects we observe in fruit flies lacking the iPLA2-VIA gene. Interestingly, mutations in the vps35 gene also cause Parkinson’s disease.”
Bellen and his colleagues confirmed these results in vertebrate neurons grown in the lab.
Connection with alpha-synuclein, a hallmark of Parkinson’s disease in vertebrate neurons
In addition, the researchers found that having high levels of alpha-synuclein leads to retromer dysfunction, lysosomal expansion and ceramide accumulation. Interestingly, these defects triggered by excess alpha-synuclein also were reduced by treatment with the same drugs that improved the defects observed in fruit flies lacking the iPLA2-VIA gene.
Altogether, this work provides new evidence suggesting connections between new and previously identified pieces known to be associated with the Parkinson’s disease puzzle and suggest a mechanism that can lead to the condition.
“We show that loss of phospholipase PLA2G6 leads to ceramide accumulation, defects in retromer function, progressive lysosome expansion and, finally, to progressive neurodegeneration with characteristics in common with those observed in Parkinson’s disease. And the same drugs that interfere with ceramide synthesis also ameliorate the condition. We also found a place for alpha-synuclein in the disease puzzle,” Lin said.
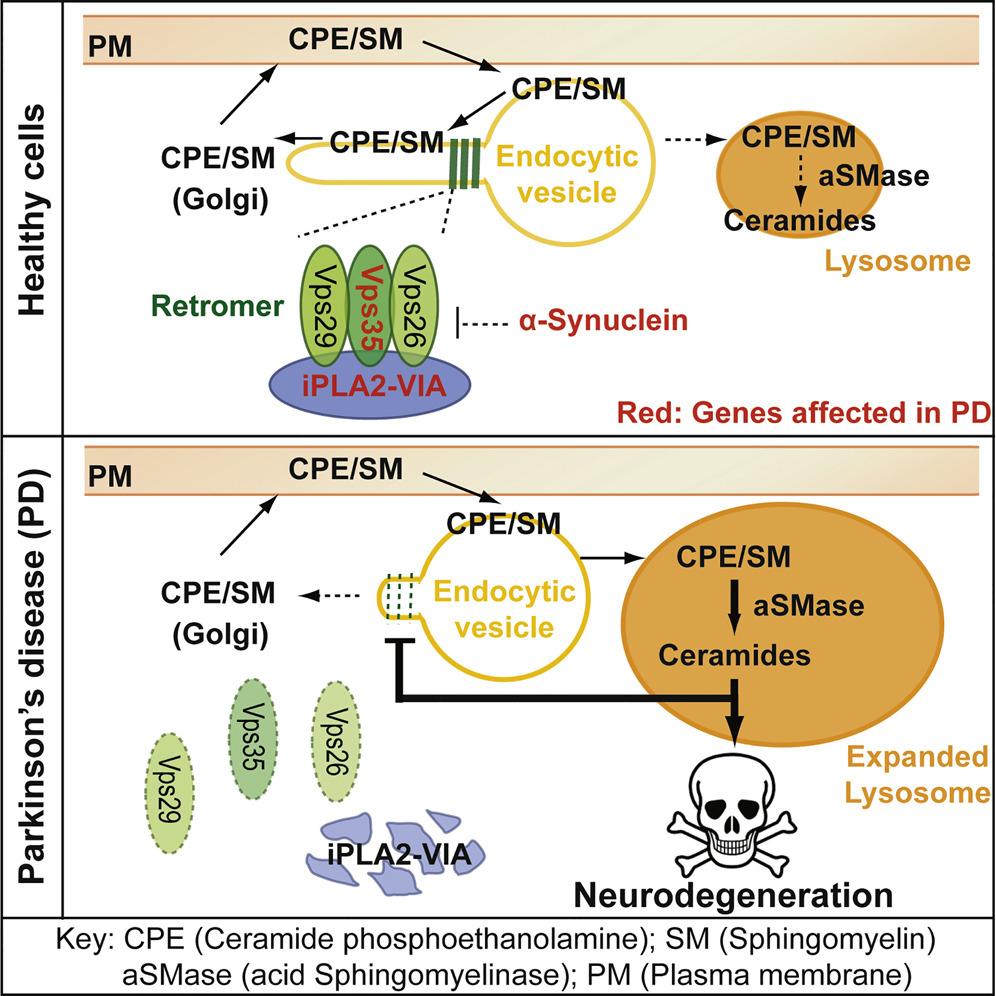
“We think that our work is important because it points to a potential mechanism leading to Parkinsonism and perhaps Parkinson’s disease,” Bellen said. “We propose that loss of phospholipase PLA2G6 or a reduction in Vps35 causes a disruption of retromer function that creates an insidious stress for the cell and is potentially at the root of these conditions. If retromer function is disrupted, neurons are not able to reuse some ceramide-derived lipids, and more of them travel to the lysosomes, which have to work harder over time and hence start expanding. Also, as ceramide-derived lipids are shuttled to the lysosomes to produce ceramides, the cell builds up ceramides, which redistribute to the cell membranes and membranes of other organelles, such as mitochondria, disrupting their functions.”
The researchers propose that the pathway becomes gradually and increasingly disturbed because of the stiffening of membranes by intercalating ceramides, which creates progressively more stress as well as mitochondrial dysfunction that eventually would lead to Parkinsonism and Parkinson’s disease in the long term.
Read all the details of this work in the journal Cell Metabolism.
Other contributors to this work include Pei-Tseng Lee, Kuchuan Chen, Dongxue Mao, Kai Li Tan, Zhongyuan Zuo, Wen-Wen Lin and Liping Wang, all at Baylor College of Medicine.
This project was supported in part by the Intellectual and Developmental Disability Research Center grant number 1U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Further support was provided by Friedreich’s Ataxia Research Alliance, Target ALS, the Huffington Foundation and the Robert A. and Renee E. Belfer Family Foundation.