Chronic jet lag leads to human liver cancer in a mouse model
 When asked about what could cause cancer, people most likely think of chemicals like tobacco or radiation such as U.V. light in sunshine, but chronic jet lag probably does not come to mind. Human epidemiological studies have linked chronic jet lag, also known as chronic circadian dysfunction, to increased liver cancer risk. However direct evidence that it leads to liver cancer has been lacking.
When asked about what could cause cancer, people most likely think of chemicals like tobacco or radiation such as U.V. light in sunshine, but chronic jet lag probably does not come to mind. Human epidemiological studies have linked chronic jet lag, also known as chronic circadian dysfunction, to increased liver cancer risk. However direct evidence that it leads to liver cancer has been lacking.
A recent study led by researchers at Baylor College of Medicine and published in the Journal of Hepatology is the first to experimentally demonstrate that chronic circadian dysfunction is indeed a human carcinogen.


“We worked with a humanized mouse model that was developed by co-author Dr. Karl Dimiter Bissig at Duke University,” said lead corresponding author Dr. Loning Fu, associate professor of medicine – gastroenterology and member of the Dan L Duncan Comprehensive Cancer Center at Baylor. “This animal model has both human and mouse liver cells in the animals’ livers, which allows us to study the effect of disrupting the circadian rhythm on cancer development in human cells.”
Circadian rhythm is the 24-hour internal timekeeper in our brain that regulates cycles of alertness, sleepiness and practically all functions of the body by being in sync with the planet’s day-and-night cycle.
Recent studies revealed that when the internal clock goes out of sync, disease has a better chance of developing.
Humanized mice were exposed to two different conditions. One group of animals was maintained in sync with the natural day-and-night cycle. For the other group, the researchers changed the light and dark periods the animals were exposed to, to create the equivalent of the changes a person experiences when flying back and forth from San Francisco to London every week for many weeks.
Chronic circadian dysfunction is a human carcinogen
We found that, compared to mice kept in normal light/dark cycles, mice in the jet-lagged group had a shorter lifespan as well as increased cirrhosis, jaundice (when skin or the white of the eyes turns yellow) and also developed cancer in both mouse and human liver cells,” Fu said. “Importantly, chronic jet lag also induced metastasis from humanized livers.”
Blood analyses and microscopy studies of the livers revealed multiple commonalities between humanized mice and patients with liver cancer, including glucose intolerance, abnormal fat accumulation in the liver, inflammation and fibrosis. This supports the validity of this model to study the human condition.
“We show that as the tumor progresses, biomarker profile and genetic expression patterns in the cells change,” Fu said.
Chronic jet-lagged humanized mice spontaneously developed liver cancer in human liver cells following the same process and molecular pathways as those in humans. Gene expression studies reveal that spontaneous cancer development in this model is driven by changes in the expression of thousands of genes which depend on cell type, time and disease stage.
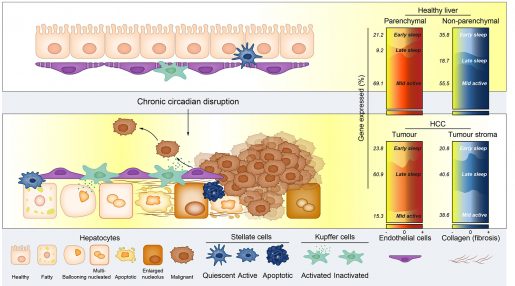

One of the important findings of the paper is that, once the tumors spontaneously develop in response to chronic circadian disruption, returning the mice to a normal circadian clock slows tumor development and prevents metastasis,” said co-corresponding author Dr. David Moore, professor and chair of the Department of Nutritional Sciences and Toxicology at the University of California, Berkeley.
“When the animals reenter normal circadian rhythm, the gene expression pattern is restored to what it was before,” Moore said.
“I am excited that our findings have significant implications at the clinic level,” Fu said. “This work contributes new knowledge that can promote the development of improved therapies for this cancer, and to better understand the mechanisms of carcinogenesis.”
Our work shows that circadian influences in cancer cannot be underestimated – chronic circadian dysfunction is a human carcinogen,” Moore said.
“The findings raise awareness of the increased cancer risk for people working night shifts for a long time or traveling across several time zones regularly, and our humanized mouse model provides a valuable tool to study this condition for which there are no effective treatments.”
Jennifer Padilla, Noha M. Osman, Beatrice Bissig-Choisat, Sandra L. Grimm, Xuan Qin, Angela M. Major, Li Yang, Dolores Lopez-Terrada, Cristian Coarfa and Feng Li also contributed to this work. The authors are affiliated with one of the following institutions: Baylor College of Medicine, Duke University or University of California, Berkeley.
This work is funded by grants from NIH/NCI (R01 CA230848 and R01 CA238988), the Cancer Prevention and Research Institute of Texas (MIRA RP150587), NIH/NIDDK (R01 DK11546, R01DK1219701), the Duke Cancer Institute (P30 CA014236), the Public Health Service Grant 506 DK56338 to TMC Digestive Diseases Center, and CPRIT (RP210227 and RP200504). Further support was provided by NIH/NCI P30 shared resource grant CA125123, NIH/NIEHS grants 1P42 ES0327725 and P30 ES030285, and NIMHD P50MD015496.
Follow From the Labs on X @BCMFromtheLabs and Instagram!