Reduced levels of FKBP5 promote atrial fibrillation
Atrial fibrillation (AFib) is the most common type of heart arrhythmia. This serious condition occurs when the heart beats so fast that the upper chambers of the heart quiver. This irregular heartbeat can lead to severe conditions, including heart failure, dementia and a fivefold increase in the risk of stroke.
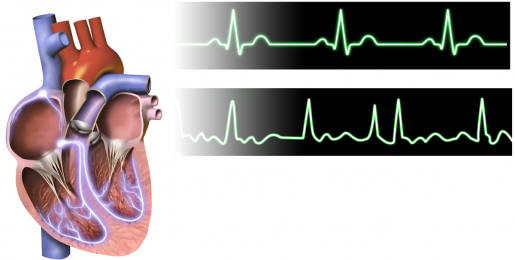
AFib affects more than 33.5 million people worldwide, and its prevalence is increasing. Despite intense research to better understand AFib, the molecular mechanism underlying the progression of this condition remains poorly understood.
At Baylor College of Medicine, Dr. Na Li’s lab and her colleagues have been investigating AFib’s underpinnings and identified key molecules and associated processes contributing to the development of persistent AFib forms. Their findings, published in the journal Circulation Research, represent a crucial step toward developing a therapeutic strategy to prevent AFib progression and maintenance.
Research on FKBP5 brings new insights

“Previous studies have shown a reduction in the expression of the FKBP5 gene in patients with AFib,” said Li, associate professor of medicine and corresponding author of the work. “Even though this gene has been well studied in brain cells, its role in the heart had not been examined before. In this study we investigated whether a reduced activity of gene FKBP5 was causally linked to AFib and delineated its function in the heart using animal models.”
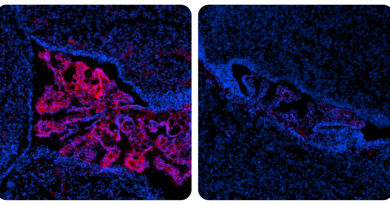
First, the researchers measured the levels of protein FKBP5 in heart samples of patients with chronic AFib. They found that the levels were significantly reduced, when compared to those in individuals without the condition.
Then, the team developed a mouse model of AFib in which the gene Fkbp5 was absent only in heart cells, called cardiomyocytes. “These Fkbp5-deficient mice had an increased susceptibility to AFib, mimicking the condition we see in patients,” Li explained. “Restoring FKBP5 protein levels specifically in the atria, the two upper heart chambers, resulted in a lower propensity to AFib compared to the Fkbp5-deficient mice.”
These studies demonstrate a causal relationship between reduced FKBP5 protein level and the enhanced AFib susceptibility.
New pieces in the AFib puzzle reveal therapeutic potential
In the animal model, the absence of FKBP5 protein promoted an increase in the level and function of another protein called NCX1 – a key ionic transporter molecule in excitable cells like cardiomyocytes. The elevated level of NCX1 in chronic AFib patients was already known; however, the molecular mechanism behind this phenomenon remained unclear prior to this study. The team discovered that the absence of FKBP5 indirectly increased NCX1 production in cardiomyocytes by enhancing the activity of the transcription factor HIF-1a, which then promoted NCX1 transcription.
“Interestingly, the increased activity of HIF-1a has been previously associated with AFib patients. Yet, for the first time, we’ve unveiled a mechanistic link between enhanced HIF-1a function and its pro-arrhythmic effect through modulation of NCX1 expression,” Li said.
More importantly, the researchers found that normalizing HIF-1a activity with compound 17-AAG in Fkbp5-deficient mice can prevent AFib, pointing to FKBP5 as a potential novel target for the treatment of this life-threatening condition.
Other contributors to this work include Xiaolei Wang, Jia Song, Yue Yuan, Luge Li, Issam Abu-Taha, Jordi Heijman, Liang Sun, Shokoufeh Dobrev, Markus Kamler, Liang Xie, Xander H.T. Wehrens, Frank T. Horrigan and Dobromir Dobrev. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine, University of Duisburg-Essen, Maastricht University, Montreal Heart Institute and Université de Montréal.
This study is supported by grants from the National Institutes of Health (R01HL136389, R01HL163277, R01HL147108, R01HL131517, R01HL089598, R01-HL160992, UM1HG006348, R01DK114356, S10OD032380 and P30CA125123). Further support was provided by the European Union (large-scale network project MAESTRIA No. 965286), the Netherlands Organization for Scientific Research (NWO/ZonMWVidi 09150171910029) and American Heart Association (23POST1013888 and EIA 936111).
Follow From the Labs on Twitter @BCMFromtheLabs.