STANN reveals new insights into how the brain functions
To better appreciate how a complex organ such as the brain functions, scientists strive to accurately understand both its detailed cellular architecture and the intercellular communications taking place within it.

At Baylor College of Medicine, Dr. Md. Abul Hassan Samee and his colleagues have taken a major step in that direction. They developed advanced computational methods that led to new insights into the intricacies of brain structure and function that may enhance the understanding of this complex organ, both in health and disease.
“Currently, we have technologies that allow us to identify and locate individual cells in a tissue. We are also capable of determining what are the products produced by each single cell in that tissue,” said corresponding author Samee, assistant professor of molecular physiology and biophysics and a member of the Center for Organ Repair and Renewal at Baylor.

Mammalian brains are complex and comprise several millions to hundreds of billions of cells, and, when analyzed, they generate vast amounts of data. The challenge has been to develop ways to integrate the information in those datasets to generate a model that reliably reflects how an organ works.
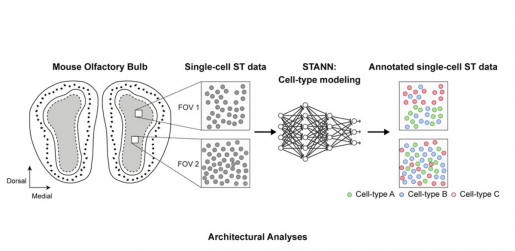
In the current study, Samee collaborated with Francisco José Grisanti Canozo, first author of the work, and Dr. James Martin, both with Baylor and the Texas Heart Institute, and Zhen Zuo, also with Baylor, to develop a neural network model to elucidate architectural and functional aspects of complex tissues. They have called the model Spatial Transcriptomics cell-types Assignment using Neural Networks (STANN).
“We also used other advanced, sophisticated computational methodologies that make the model more rigorous,” Samee said. “We applied STANN and the other methods to existing brain datasets of the mouse olfactory bulb and started to see very interesting patterns in the brain’s cellular architecture and functionality.”
The brain comprises distinct morphological layers, and STANN allowed the researchers to predict a detailed picture of their cellular organization. “Layer by layer, our model provided the precise location of different cell types, whether they communicated with each other and by which means,” Samee said. “This was a ‘eureka moment’ for us.”
Samee and his colleagues determined that the cell-type composition is quite consistent within a morphological layer. For example, a particular layer may have a certain percentage of astrocytes, neurons and microglia that remains the same throughout the same layer. “If we take several small sections from different areas of the same morphological layer, these percentages look very similar to each other. However, the percentages may change from one layer to another,” Samee explained.
The team saw a different pattern emerge when they looked at cell colocalization. “For instance, in one area of a morphological layer we may see astrocytes colocalize with olfactory neurons. But in another area from the same morphological layer these cells can be completely separated,” Samee explained. “We also see that intercellular communication between two cell types changes in different areas of a morphological layer, a reflection that gene regulatory networks are changing with location.”
The researchers hypothesize that morphological layers in the brain have different spatially localized communities of cell types. The communities are similar in cell type composition, but there are large differences in how cell types colocalize and communicate within a community. This suggests that brain cell types have spatially localized subtypes performing location-specific functions.
This new detailed view of brain organization at the single-cell and functional levels had not been described before,” Samee said.

“The neural network model approach we have developed in this work presents an ‘instruction manual’ for other researchers to use to study other areas of the brain or other organs, such as the heart, the main interest of my lab,” said co-author Martin, vice chairman and professor of molecular physiology and biophysics, Vivian L. Smith Chair in Regenerative Medicine and director of the Center for Organ Repair and Renewal at Baylor College of Medicine. Martin also is the director of the Cardiomyocyte Renewal Laboratory at the Texas Heart Institute.
Read all the details of this study in the journal Cell Systems.
This work was supported by grants from the National Institutes of Health (HL127717, HL130804 and HL118761), Vivian L. Smith Foundation, State of Texas funding and Foundation Leducq Transatlantic Networks of Excellence in Cardiovascular Research (14CVD01).



