Taking a closer look into the tumor microenvironment with nano-radiomics
A tumor mass consists not only of cancer cells but also a variety of host cells, secreted factors and extracellular matrix proteins such as collagen, that together form the tumor microenvironment (TME). Research has shown that the TME can participate in the fight against cancer, but also can promote its growth and help the tumor evade the immune response.
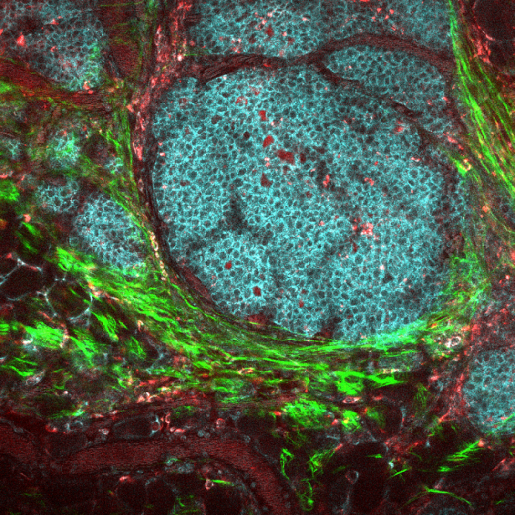
Scientists are now developing cellular immunotherapies that attempt not only to promote the anti-cancer activity of the immune system, but also combat the inhibitory effect of the tumor microenvironment. While it is straightforward to assess the effect of new therapies on cancer cells, assessing the effectiveness on the TME is challenging.
“Understanding the response of the TME to anti-cancer therapy is becoming increasingly important,” said co-corresponding author Dr. Robin Parihar, assistant professor of pediatric hematology-oncology at Baylor College of Medicine and Texas Children’s Hospital and a member of Baylor’s Center for Cell and Gene Therapy. “This understanding is essential when evaluating the effect of therapies attempting to counter the cancer-promoting activity of the TME.”

Currently, imaging technologies such as computed tomography (CT) or magnetic resonance imaging (MRI) generate three-dimensional images that provide information about the overall tumor response to therapy. For instance, they can show whether the tumor is growing or shrinking, but provide very little, if any, information about the TME, which represents a minor part of the tumor mass.
Parihar approached Dr. Ketan Ghaghada, assistant professor of radiology at Baylor and a member of the Translational Imaging Group (TIGr) at Texas Children’s, and their laboratories began a collaboration to develop a noninvasive method to assess the effect of a cellular immunotherapy treatment specifically directed at the TME.
Nano-radiomics unveils treatment effect on the tumor microenvironment
Parihar, Ghaghada and their colleagues developed nano-radiomics, a novel method that combines imaging technology using a nanoparticle contrast agent, with radiomics for computational mining of 3-D imaging data.

“Radiomics is an emerging area in the field of radiology wherein images are analyzed to extract information that may reveal patterns or textures in the tumor that are not visible to the naked eye. To enhance the quality of the images, we used a nanoparticle contrast agent developed in our lab that has a different pattern of distribution within the tumor than traditional contrast agents,” said Ghaghada, co-corresponding author of this work and a member of Baylor’s Dan L Duncan Comprehensive Cancer Center. “We assessed whether a non-invasive approach that combined the nanoparticle contrast agent with radiomics analysis would enable us to see changes in TME after treating it with immunotherapy.”
Using mouse models of cancer, the researchers treated one group of animals with a cellular immunotherapy that effectively eliminated the TME and another group with a placebo. Both groups received the nanoparticle contrast agent followed by a CT scan. Radiomics analysis of the imaging data of both groups revealed texture-based features that distinguished the two groups while traditional CT scans did not.
These findings suggested that this new approach has the potential to enhance the ability of clinicians to noninvasively assess the effect of treatments directed at the TME, ultimately enhancing the results of cancer treatment and management.
Find all the details of this study in the journal Science Advances.
Other contributors to this work include Laxman Devkota, Zbigniew Starosolski, Charlotte H. Rivas, Igor Stupin and Ananth Annapragada. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine, Texas Children’s Hospital and Houston Methodist Hospital.
This work was supported by funding from Texas Children’s Hospital Pediatric Pilot Research Award, American Cancer Society (MRSG-16-196-01-LIB), and the NIH (NCI/NIDCR 1U01DE028233-01).