NFIA gene reveals a new facet of astrocyte function
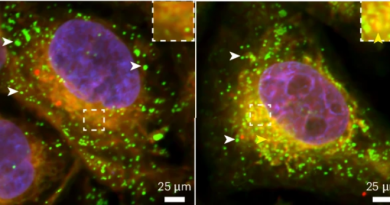
Astrocytes are no longer seen only as supporting cells of the healthy adult brain. Increasing experimental evidence not only shows that they are a diverse population of cells, but also that they are directly involved in a wide variety of complex and essential functions, including neuronal communication through synapses and regulation of neural circuit functions.
In his laboratory at Baylor College of Medicine, Dr. Benjamin Deneen, professor of neurosurgery and a member of the Center for Stem Cell and Regenerative Medicine, and his colleagues, are interested in learning what these previously underappreciated cells can do for the adult brain. In this study, they reveal a new role astrocytes play in normal brain function.
“Previous work showed that astrocytes comprise diverse populations with unique cellular, molecular and functional properties. They occupy distinct brain regions, indicating regional specialization,” said Deneen, who holds the Dr. Russell J. and Marian K. Blattner Chair and is a member of the Dan L Duncan Comprehensive Cancer Center at Baylor.

There is evidence suggesting that transcription factors – proteins involved in controlling gene expression – regulate astrocyte diversity. Deneen and his colleagues looked to get a better understanding of the role transcription factor NFIA, a known regulator of astrocyte development, played in adult mouse brain functions.
The NFIA gene is key to astrocytes
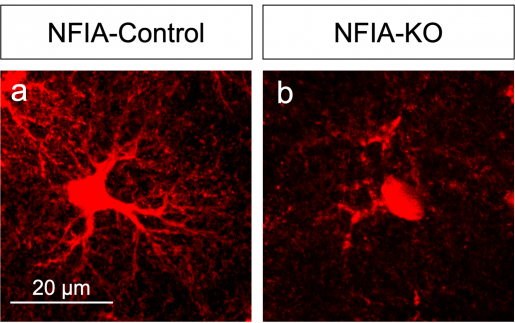
The researchers worked with a mouse model they had genetically engineered to lack the NFIA gene specifically in adult astrocytes in the entire brain. But, when they analyzed several brain regions, looking for alterations in astrocyte morphology, physiology and gene expression signatures, they were surprised by unexpected findings.
“We found that NFIA-deficient astrocytes presented defective shapes and altered functions,” Deneen said. “Surprisingly, although the NFIA gene was eliminated in all brain regions, only the astrocytes in the hippocampus were severely altered. Other regions, such as the cortex and the brain stem, were not affected.”
Astrocytes in the hippocampus also had less calcium activity – calcium is an indicator of astrocyte function – as well as a reduced ability to detect neurotransmitters released by neurons. NFIA-deficient astrocytes also were not as closely associated with neurons as normal astrocytes.
Importantly, all these morphological and functional alterations were linked to defects in the animals’ ability to learn and remember, providing the first evidence that astrocytes are to some extent controlling the neuronal circuits that mediate learning and memory.
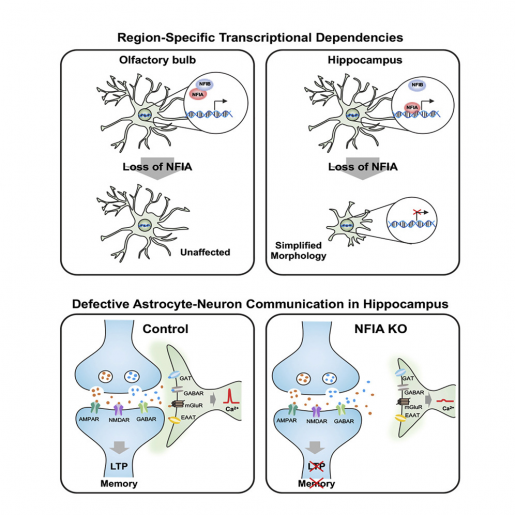
“Astrocytes in the brain are physically close to and communicate with neurons. Neurons release molecules that astrocytes can detect and respond to,” Deneen said. “We propose that NFIA-deficient astrocytes are not able to ‘listen’ to neurons as well as normal astrocytes, and, therefore, they cannot respond appropriately by providing the support needed for efficient memory circuit function and neuronal transmission. Consequently, the circuit is disrupted, leading to impaired learning and memory.”
Interested in all the details of this work? Find them in the journal Neuron.
Other contributors to this work include first author Anna Yu-Szu Huang, Junsung Woo, Debosmita Sardar, Brittney Lozzi, Chia-Ching John Lin, Daniela Felice, all at Baylor, and Adriana Paulucci-Holthauzen at MD Anderson Cancer Center.
This work was supported by grants from the National Institutes of Health (NINDS NS071153 and NIMH AG054111) and the National Multiple Sclerosis Society (RG-1501-02756). This project was also supported in part by IDDRC grant number 1U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (P30 AI036211, P30 CA125123, and S10 RR024574).