Zooming in on cilia sheds light into blinding diseases
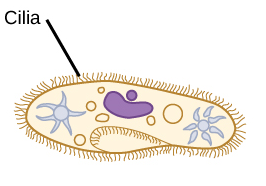
Cilia are short hair-like structures found on the surface of most cells. They play important roles in human and animal development and in every day cellular activities.
In the human eye, cilia are particularly important in vision because they work as light sensors and defects in their structure can lead to retinal degeneration and loss of vision.
“There is a large group of diseases called ciliopathies that are caused by genetic defects in the components of the structure of cilia. One the most common symptoms of ciliopathies is retinal degeneration and blindness, such as seen in retinitis pigmentosa and Bardet-Biedl syndrome,” said Dr. Theodore G. Wensel, professor and Robert A. Welch Chair in Chemistry in the Department of Biochemistry and Molecular Biology at Baylor College of Medicine.

In his lab at Baylor, Wensel and his colleagues investigate how genetic defects linked to retinal ciliopathies affect the structure of the cilia. But cilia are tiny, and the methods that have traditionally been used to study structural changes do not have enough resolution to reveal details of cilia internal structure.
In the current study, Wensel and his colleagues reported the development and application of new imaging methods that provided a higher resolution view of the internal structure of normal and diseased retinal cilia.
Zooming in on cilia
“We wanted to look inside cilia to see how the molecules known to be involved in the disease caused the defects,” Wensel said. “We developed new methods – cryoelectron tomography and STORM (super-resolution stochastic optical reconstruction microscopy) – that provided a nanoscale view of the internal structure of the cilia. We worked with normal mice and mouse models of the human disease, Bardet-Biedl syndrome.”
The researchers applied these methods first to determine the structure of cilia in normal retina and found sub-compartments that had not been seen before. When they applied their higher resolution techniques to diseased retinas, they were able to pinpoint a more precise location of proteins associated with the condition.
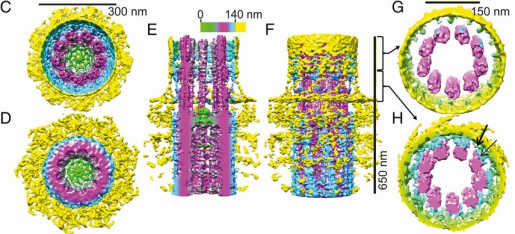
Before, we could say that a particular protein encoded by a disease gene was located at the cilium. Now we can say that it is located in the center of the axon, which is a tiny structure located in the center of the cilium, or in another of the newly discovered compartments,” Wensel said.
The researchers also discovered that some of the genetic defects alter the location of some of the proteins in the structure, and this information can potentially be useful to design therapies.
“Our findings provide more details of the structural changes associated with cilia defects in Bardet-Biedl syndrome. Our hope is that by understanding the disease mechanisms better we can develop therapies for this and other related conditions,” Wensel said.
Interested in reading all the details of this work? Find it in the Proceedings of the National Academy of Sciences.
Other contributors to this work include first author Michael A. Robichaux, Valencia L. Potter, Zhixian Zhang, Feng He, Jun Liu and Michael F. Schmid.
This study was supported by NIH Grants R01-EY007981 and R01-EY026545, the Welch Foundation (Q0035 and AU-1714), the National Center for Macromolecular Imaging (P41-GM103832), NIH Fellowships F31-EY028025 and F32-EY027171. Further support was provided by the Baylor College of Medicine Vision Research Core (P30-EY002520).