Beyond the airway and into the brain, yeast impairs memory in mice
An increasing number of clinical observations indicates that fungi are becoming a more common cause of upper airway allergic diseases such as asthma, as well as other conditions such as sepsis, a potentially life-threatening disease caused by the body’s response to an infection. Importantly, explains Dr. David B. Corry, fungal infections causing airway allergic diseases and sepsis have been associated with increased risk for dementia later.
“These observations connecting fungal infections with neurological impairments led us to investigate the possibility that fungus might produce a brain infection and, if so, the consequences of having that kind of infection,” said Corry, who is professor of medicine-immunology, allergy and rheumatology and Fulbright Endowed Chair in Pathology at Baylor College of Medicine.

The researchers began their investigation by developing a mouse model of a low-grade fungus infection with the common yeast C. albicans that would not cause severe disease, but might carry implications for brain function. They tested several doses and finally settled on one dose of 25,000 yeasts.
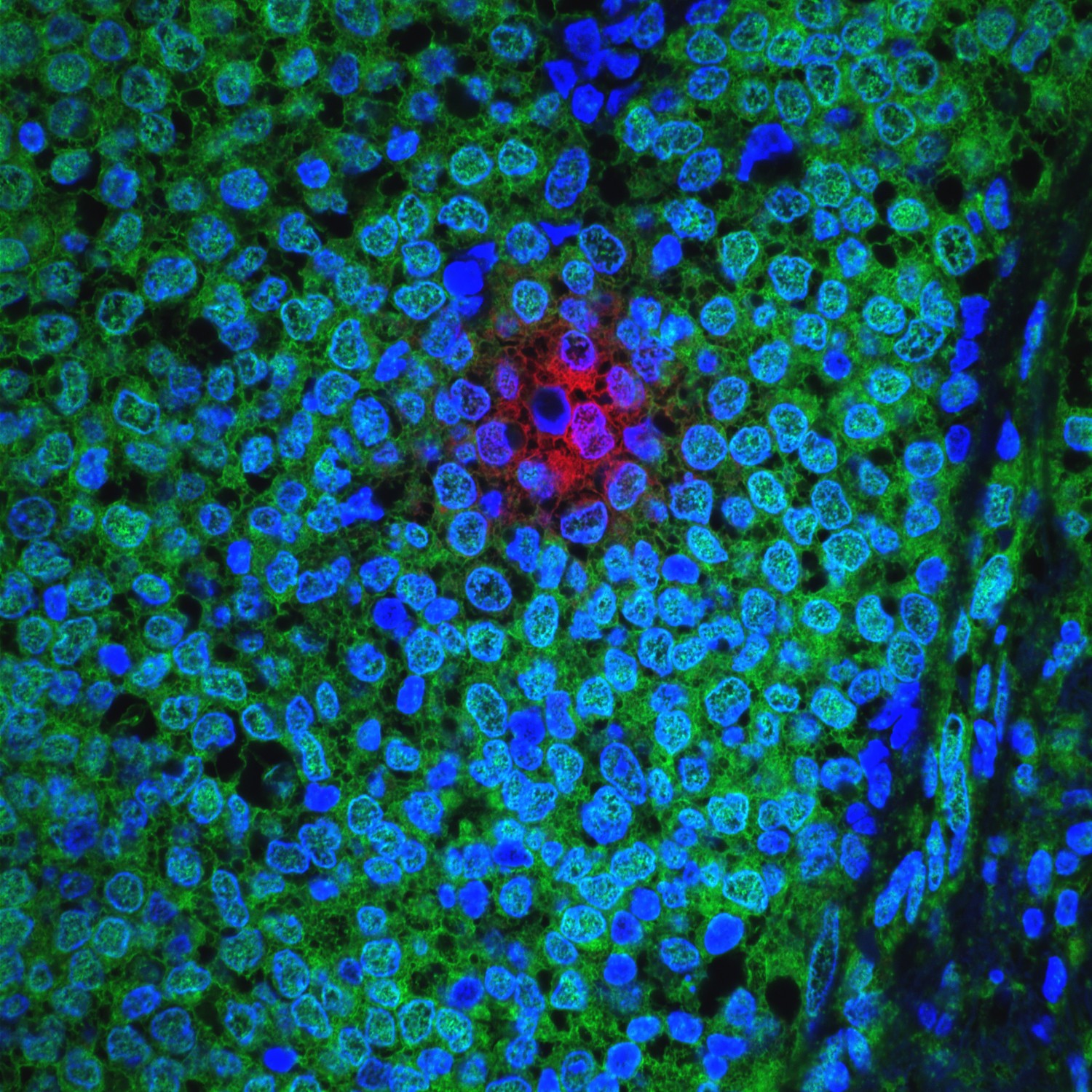
They injected C. albicans into the bloodstream of mice and were surprised to discover that the yeast can cross the blood-brain barrier, a robust protective mechanism the brain employs to exclude all kinds of large and small molecules, as well as a number of microorganisms that can potentially damage the brain.
“We thought that yeast would not enter the brain, but it does,” said Corry, who also is a member of the Dan L Duncan Comprehensive Cancer Center. “In the brain, the yeast triggered the activity of microglia, a resident type of immune cell. The cells became very active ‘eating and digesting’ the yeast. They also produced a number of molecules that mediated an inflammatory response leading to the capture of the yeasts inside a granule-type structure inside the brain. We called it fungus-induced glial granuloma, or FIGG.”
C. albicans and Alzheimer’s disease
Corry and his colleagues also tested the animals’ memory in both yeast-infected and non-infected mice. They found that infected mice had impaired spatial memory, which reversed when the infection cleared.
The mice cleared the yeast infection in about 10 days; however, the microglia remained active and the FIGGs persisted well past this point, out to at least day 21. Intriguingly, as the FIGGs formed, amyloid precursor proteins accumulated within the periphery and amyloid beta molecules built up around yeast cells captured at the center of FIGGs. These amyloid molecules are typically found in plaques that are the trademark of Alzheimer’s disease.
“These findings suggest that the role fungi play in human illness potentially goes well beyond allergic airway disease or sepsis,” Corry said.
The results prompted us to consider the possibility that in some cases, fungi also could be involved in the development of chronic neurodegenerative disorders, such as Alzheimer’s, Parkinson’s and multiple sclerosis. We are currently exploring this possibility.”
“For these reasons, if we better understand how our immune system deals with this kind of constant threat and what are the weaknesses in our immunological armor that occur with aging allowing fungal disease to take root, then we would likely increase the possibility of finding ways to fight back,” Corry said.
Interested in reading all the details of this work? Find it in Nature Communications.
Other authors who contributed to this work include Yifan Wu, Shuqi Du, Jennifer L. Johnson, Hui-Ying Tung, Cameron T Landers, Yuwei Liu, Brittany G. Seman, Robert T. Wheeler, Mauro Costa-Mattioli, Farrah Kheradmand and Hui Zheng. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine, Michael E. DeBakey VA Center for Translational Research on Inflammatory Diseases and the University of Maine.
This work was supported by the US National Institutes of Health (grants P30684 AI036211, P30 CA125123, S10 RR024574, T32AI053831, R01HL117181, R01 AI135803 and R41AI124997) and the VA Office of Research and Development (grant 5I01BX002221).