Study identifies a new rare genetic syndrome associated with severe infections and lung disease in infants
An international team of researchers has identified a new rare genetic condition – a chromosome breakage syndrome associated with severe infections and lung disease in infants. The discovery provides an explanation for this deadly pulmonary disease, possibilities to diagnose it and opportunities for developing alternate ways to treat it. The results appear July 18 in the Journal of Clinical Investigation.
Independently from each other, a team of researchers at Baylor College of Medicine and a team at the University Medical Center Utrecht in The Netherlands identified a local family with two young children with severe infections and lung disease. Dr. Saskia N. van der Crabben, co-first author and, at the time, a resident in clinical genetics at UMC Utrecht, was involved in patient care of both Dutch siblings. “I ordered diagnostics, counseled the family, coordinated interdisciplinary clinical collaboration and later on proposed this case as a research project,” said van der Crabben.
Co-first author Dr. Marije Hennus, a pediatric intensive care doctor at UMC Utrecht, was the primary physician for both siblings. “Having had the eldest child under my direct care I was shocked to have her sibling admitted with an identical course of disease only two years later. This triggered my belief that an underlying genetic defect was likely at the heart of their problems,” said Hennus.
“Dr. Hennus and I were dedicated to finding an answer for this family. I am very glad we finally found it,” said van der Crabben.
“The project ‘grabbed me’ when I heard of a family that had lost two young children to a rare lung disease; both siblings were approximately the same age when they passed away. Because this happened twice in the same family, our odds of finding a genetic cause increased significantly,” said co-senior author Dr. Gijs van Haaften, associate professor of genetics and leader of the group in Utrecht.

In the meantime, the group at Baylor found out about a similar family through the genetics consult service. The physicians at Texas Children’s Hospital caring subsequently for two siblings, both having severe problems with lung disease and infection, had asked the genetics consult service to see them. The family was presented as an unknown disorder by then genetics fellow and co-author, Dr. Sandesh C.S. Nagamani, (now assistant professor at Baylor) at Genetics Clinical Rounds attended by co-senior author Dr. Sharon Plon professor of pediatrics – oncology and molecular and human genetics at Baylor and director of the Cancer Genetics Clinical and Research Programs at Texas Children’s Hospital. “I mentioned to the genetics consult team that having problems with handling DNA damage could be the cause of the infants’ clinical problems,” said Plon.
“They sent off a clinical test that looked at the infant’s ability to respond to DNA damage and found the result to be highly abnormal,” said Plon. “At that point I talked to the family about their interest in entering our research study which is designed to use new genomic sequencing methods to try to understand these kind of disorders. The parents agreed for the children and themselves to enter the study.”
The search for the gene
The Baylor and the Utrecht teams continued working independently trying to identify the gene that could explain the infants’ condition. The process involved first sequencing the DNA of the children and then comparing their genes with those listed in databases. “A lot of our work is to try to figure out what rare genetic change in is in this family that isn’t found in other individuals,” said Plon.
The first attempts at finding the gene resulted in a fairly long list of gene candidates. The genetic databases at the time did not have enough information about the genes and their variants present in normal populations. But “about a year and a half ago a new member of the lab, Deb Ritter, went back and reanalyzed the data using some of the newer databases of normal individuals as a comparison and she got it down to two genes,” said Plon. The new databases contained genetic information from tens of thousands of individuals.
“I did the bioinformatics analysis,” said co-first author Dr. Deborah Ritter, research scientist of pediatrics at the Human Genome Sequencing Center at Baylor and Texas Children’s Hospital. In collaboration with Dr. David Wheeler, professor of molecular and human genetics at Baylor, Ritter compared the children’s genes with those in “large databases of healthy individuals so we could look for truly rare mutations in the children’s DNA.”
The gene they identified, called NSMCE3, “is a very small gene; it was extremely unique that two rare mutations were present in it,” said Ritter.
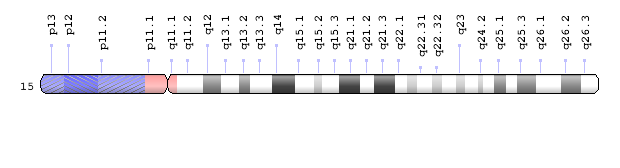
Ritter’s work strongly suggested that the mutations of NSMCE3 gene present in the infants were responsible for the severe lung disease and infections that affected the children. “Combined with the fact that there were no other very compelling mutations, and the literature on this gene made sense for a role in DNA repair, then the case for this gene being causal was very strong,” said Ritter. Nevertheless, “we really needed to find additional families; it’s very hard to prove a case with a single family,” said Plon.
The Utrecht team had identified the same gene in the family they were working with. “We found a candidate gene with mutations in it, and functional work in collaboration with experts in the University of Sussex in the UK confirmed the devastating effects of the mutations,” said van Haaften. To find additional families “we entered the candidate gene in ‘GeneMatcher,’ a clever webtool developed by Johns Hopkins University for rare disease researchers, and found out the group of Dr. Plon also was interested in the gene.”
Dr. Plon had also entered her team’s gene in GeneMatcher. “Originally there were no matches. Nobody was interested in this gene, and then, six months later, we got an email saying ‘you’ve got a match,’” said Plon. “I sent a simple email to van Haaften saying that we had a family with two children with severe infections, and two mutations in this gene. Dr. van Haaften immediately replied, ‘it sounds very similar to our family.’ So we decided to collaborate.”
A long-term fruitful collaboration
“By combining our data we had very strong evidence that the mutations in the candidate gene were indeed responsible for the disease,” said van Haaften. The Dutch family “has exactly the same mutation as one of the two mutations in our family,” said Plon.
Sadly, all four children, two in the Dutch family and two in the American family, have died very young of this severe lung disease and infection. They also presented with chromosome breakage. According to these clinical and genetic features, the scientists named the condition ‘severe lung disease and immunodeficiency chromosome breakage syndrome.’
The mutations in the NSMCE3 gene affect a protein that is essential for repairing damaged DNA and participates in the process that separates chromosomes when cells divide. Co-senior author Dr. Johanne Murray, reader at the Genome Damage & Stability Center at University of Sussex, and co-first author, graduate student Grant McGregor also at Genome Stability, studied in detail what these specific mutations do and how they impact the function of the protein. “We were able to coordinate with other scientists – co-authors Dr. Laurence Pearl and Dr. Antony Oliver at Sussex and Dr. Jan Paleček at Masaryk University in Brno, Czech Republic – to build a picture of what is happening in the patients’ cells,” said Murray.
The mutated proteins were different from the normal protein by a single change in their amino acids, the building blocks of proteins. The single change resulted in a destabilized protein that could not carry out its normal function during DNA repair. “We were able to show that the cells were most sensitive to problems that occur when the DNA is copied,” said McGregor. In laboratory experiments in which cells were irradiated, for instance, Murray and McGregor observed that, after irradiation, cells with the mutations did not repair their DNA as well as cells without the mutations.
Because infections were common in the children with this syndrome, both teams looked into the children’s immune response. At Baylor, co-authors Dr. Jordan Orange, professor of pediatrics – rheumatology, and Dr. Ivan Chinn, assistant professor of pediatrics – allergy & immunology, studied the numbers and function of T and B cells, two important cell types in the immune response. “The patients had low percentages of T cells and poor to absent T cell responses to infectious agents. Their B cell numbers did not appear to be universally low, but we saw evidence of B cell dysfunction in all of the patients who were tested,” said Orange and Chinn. T and B cells also showed evidence of multiple chromosome breakages.
Thanks to this long-tern, international collaboration, the researchers have determined that a rare fatal syndrome characterized by chromosome breakage, infections and severe pulmonary disease is caused by inheriting a mutation in both copies of the gene NSMCE3. They identified two single mutations that can cause the syndrome. The mutated genes produce a protein that destabilizes the process of DNA repair, which leads to chromosome breakages. In addition, affected T and B cells do not perform their immunological functions properly.
Implications of this research and the future
For researchers, to describe a new genetic disorder is a significant contribution to the field. But the authors remark that what they are most excited about this work is that “now we can provide a scientific explanation to the families, hopefully improve diagnostic opportunities for future children with a similar disease and understand more of the function of the DNA repair complex in the human body in health and disease,” said van Haaften.
“This is a particularly good example of different groups doing genomic sequencing and individually not having enough data, but by sharing data achieving important results,” said Plon. “I work on the development of databases to facilitate sharing of data. It was personally rewarding to use one of those tools to enable us to define this rare disease.”
“I hope that, after publication of the paper, more patients will be identified and we will be able to get a better idea of the characteristics of the syndrome. Maybe from this new chromosomal breakage syndrome we will learn more about other breakage syndromes,” said van der Crabben.

“This was not a disease that could have been prevented, influenced by something the parents worried they did wrong or cured at this moment,” said Hennus. “I’m by far most excited that we were finally able to give the grieving families the answers and counseling they were looking for.”
For the parents of the American children who contributed samples to this study, getting the results of the research was significant. “It was a gift to us because most parents of children with unknown illnesses really never receive an answer to the question, why did my children get sick? Being able to get the answer was also a gift to the nurses, the physicians, the staff and the researchers at Texas Children’s. Texas Children’s is an incredible hospital where they see many kids from all over the world. To actually receive a specific answer to an unknown illness is very unique, very rare. For us, it was amazing,” said the father of the American children who were part of this study.
The researchers are deeply thankful to the parents of the children affected by this syndrome for participating in the study. “I cannot say enough how highly I regard their decision to participate and what an impact it has had on understanding this syndrome better, or how often I thought of their families as this work developed,” said Ritter. “Even though I only ‘met’ their DNA, the parents and the children they lost were present to me.”
###
Other authors who contributed to this work include Owen S. Wells, Magdalena Harakalova, Aaron Alt, Lucie Vondrova, Ron Hochstenbach, Joris M. van Montfrans, Suzanne W. Terheggen-Lagro, Stef van Lieshout, Markus J. van Roosmalen, Ivo Renkens, Karen Duran, Isaac J. Nijman, Wigard P. Kloosterman, Eric Hennekam, Peter M. van Hasselt, David A. Wheeler and Alan R. Lehmann from the University Medical Center Utrecht, the University of Sussex, Baylor College of Medicine and Masaryk University.
The research was supported by MRC grants G0901011, G1001668 and G1100074; CSF grant GA13-00774S and European Regional Development Fund CZ.1.05/1.1.00/02.0068; the Cancer Prevention Research Institute of Texas grant RP10189, NIH R01-CA138836 and the National Institute of General Medical Sciences and Institutional Research and Academic Career Development K12 GM084897-06. Also, The Doris Duke Charitable Foundation (DDCF) Clinical Scientist Development Award, DDCF grant 2013095 and Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (IDDRC) grant 1 U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.