New study helps researchers engineer better gene therapies
Gene therapy, a technique that is revolutionizing the treatment of multiple genetic conditions, including eye and muscle diseases and blood disorders, requires efficient and specific delivery of the genetic material to the tissue and cell type of interest. To help improve gene therapy, a multidisciplinary team led by researchers at Baylor College of Medicine, the Jackson Laboratory and the University of Massachusetts Medical School has generated a comprehensive atlas that researchers can use to select the most effective viral vehicle for their target organ. The study appeared in Molecular Therapy.

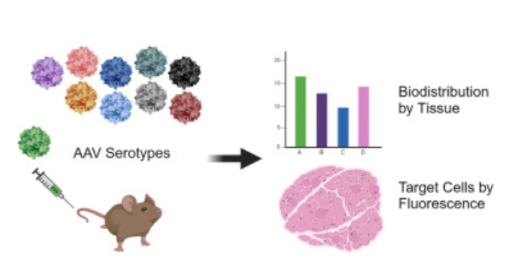
“Over the last three decades, adeno-associated viruses (AAVs) have emerged as the leading gene delivery system in mice and people, due to their efficiency and favorable safety profile,” said first author Dr. Christopher J. Walkey, assistant professor in integrative physiology at Baylor. “To assist researchers in the selection of the optimal AAV vector for their application, we have generated a detailed map of viral delivery to tissues in mice, the most commonly used experimental animal for preclinical studies.”
A much needed research tool
With this information in hand, researchers who, for instance, are developing a gene therapy to treat a muscle condition, could use the atlas to identify AAV vectors that preferentially target the muscle. Importantly, the atlas also lets researchers know if a vector is going to other tissues they do not want to target. This is important to reduce the risk of unwanted side effects of a gene therapy

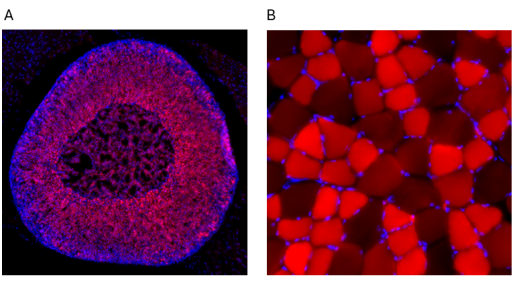
This study expands on prior work in several important ways. “We analyzed more AAVs and tissues than those that have been studied before – 10 distinct types of AAVs in 22 different tissues, both in males and females,” said corresponding author Dr. William Lagor, Kyle and Josephine Morrow Endowed Professor of integrative physiology at Baylor. “We also used a fluorescent imaging technique in organ sections to determine how efficient gene delivery was in different tissues, down to the level of the individual cells.”
New findings offer improved options for gene therapy
The approach led the researchers to discover new insights on AAV biology, some of which are relevant to potential clinical applications.
For instance, the team discovered that AAV4, a viral vector that has not been studied extensively, efficiently delivers its genetic payload to endothelial cells in blood vessels and beta cells in the pancreas. AAV4 also mostly avoids the liver, a major target organ for most of the other common AAV varieties.
“These findings with AAV4 open the door for gene therapies directed at vascular tissues, which have not yet been successful,” Walkey said. “And the affinity of AAV4 toward beta cells in the pancreas, the producers of insulin, makes it a potential candidate for gene therapy for diabetes.”
We hope that this resource, which is publicly available here, will help researchers engineer better gene therapy vectors for human conditions,” Lagor said.
“This will be useful across many disciplines that rely on AAV vectors for gene delivery in basic research. It will also make preclinical gene therapy studies in mice more efficient and reproducible, given that a lot of the homework on the best AAV for a given cell type has already been done.”
This work was a large collaborative effort from three different groups who were brought together under Phase I of the NIH’s Somatic Cell Genome Editing Consortium. Notably, the AAV were designed and produced by Dan Wang, Jote Bulcha, and Guangping Gao at UMass Med. A group at Jackson Labs in Maine, including Kathy Snow, Cathleen Lutz, and Steve Murray performed most of the fluorescent imaging experiments. Researchers at Baylor, including William Lagor, Alexa Martinez, Cecilia Ljungberg, Jason Heaney, Mary Dickinson, Denise Lanza and Christopher Walkey, investigated the distribution of the AAV vectors across different tissues.
“This project would not have been possible without such an outstanding team of collaborators,” Dr. Lagor said. “It is a great example of the value of teamwork and replication in science. We were able to readily confirm the results of our colleagues at Jackson Labs, which really improved our confidence in the findings, and vice versa. We appreciate the critical role NIH played in supporting this important project.”
This work was made possible by NIH grants (U42OD026645, U42OD035581, U42OD026635, UG3HL147367, UG3HL151545, R01HL132840, R01DK124477, R01DK136694, R56DK128098, K01DK128226, CA125123 and RR024574). Further support was provided by the JAX Cancer Center (P30CA034196), a grant from Baylor College of Medicine Intellectual and Developmental Disabilities Center (IDDRC) (P50HD103555), a CPRIT Core Facility Support Award (CPRIT-RP180672) and the Quantitative Science Shared Resource at BCM (grant P30CA125123).