Molecular profiling of meningioma can lead to improved prognosis and therapy
It has not been easy to reliably predict whether a meningioma, the most common primary brain tumor, will recur or remain benign. At Baylor College of Medicine and the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) at Texas Children’s Hospital, Dr. Akash J. Patel and his colleagues are continuing their efforts to improve tumor prognostication.
The need for improvement
The current classification is the World Health Organization (W.H.O.) system, which divides meningiomas into three categories: W.H.O. grade I (benign), grade II (atypical) and grade III (malignant).
“The W.H.O. system relies on tumor histopathology; that is, the appearance of tumor cells under the microscope. Although this system has some success predicting tumor recurrence, there is room for improvement,” said Patel, a neurosurgeon specialized in treating meningioma at Baylor St. Luke’s Medical Center and the co-corresponding author of this work.

“For instance, W.H.O. grade II and III tumors tend to recur, but we also have seen patients with grade II meningioma whose tumors have a more benign course,” said Patel, who also is an associate professor of neurosurgery at Baylor and an investigator at the Duncan NRI. “On the other hand, a portion of grade I tumors, which are generally cured with surgery, recur despite complete resection and benign microscopic features. It seems that the way we are looking at meningioma could benefit from reevaluation, and this inspired us to continue searching for evidence supporting a better way to predict tumor behavior.”
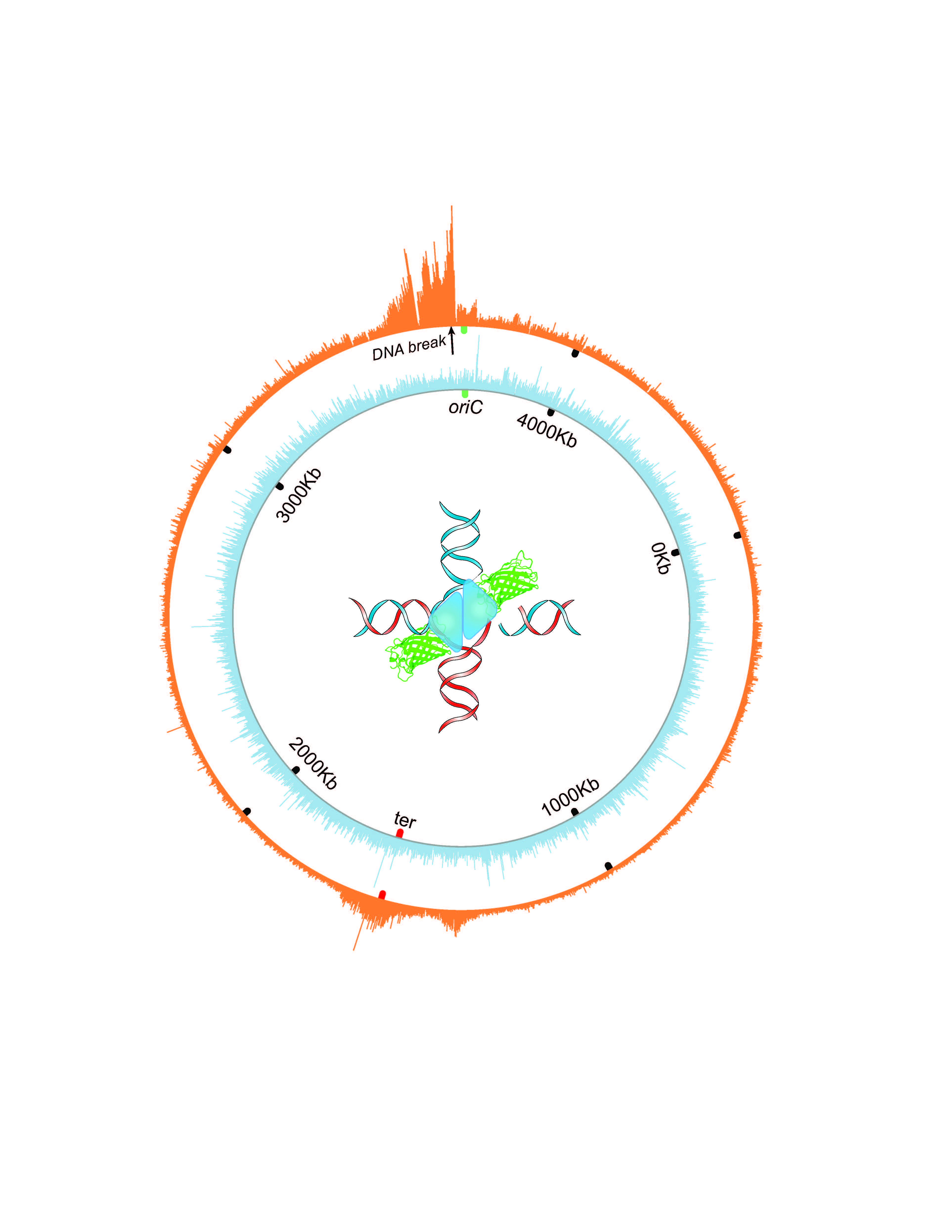
In their previous work, Patel and his colleagues examined meningioma tumors at the genetic rather than cellular level. They used three molecular profiling approaches: whole exome sequencing, RNA sequencing and gene copy number variations. They identified three distinct groups of meningioma – A, B and C – that didn’t correspond with the W.H.O. grading system. Group C tumors had a remarkably worse clinical course than group A or B tumors. These three types enabled better prediction of tumor behavior than the W.H.O. system.
In the current study, Patel and his colleagues compared their meningioma molecular profiling approach with others that used DNA methylation to classify tumors. A DNA methylation profile refers to the distribution of the chemical methyl tags along the genome, which affects which genes are expressed.
“We conducted DNA methylation profiling of the tumors we had already looked at in our previous study to determine whether these different methodologies for classifying tumors were finding the same biological groups,” Patel said.
We found that, regardless of what profiling approach we used – gene expression or DNA methylation – we always found the same molecular groups.”
In the second part of the study, the researchers investigated what was having the dominant effect on the gene expression profile in these groups. Was it DNA methylation or gene copy number variation predominantly influencing gene expression?
“To determine which one of the two had a dominant effect, we created a mathematical model, the partial least squares regression model. First author Dr. Jim Bayley, a resident in the lab, spent a year on this work,” Patel said.
With this model, the researchers can say that, for a particular gene, its expression profile is highly correlated with its methylation profile, which suggests that the expression of that gene is probably regulated by methylation. For other genes, the gene’s expression profile may be regulated by copy number variation, not by methylation.
Finally, the team wanted to understand what was driving the gene expression profile of the malignant group C tumors, the ones with the worst outcomes.
Understanding the worst tumors better
“We found that the more aggressive tumors tended to be more influenced by gene copy number losses, particularly chromosome 1p loss, which we see in the vast majority of the group C tumors,” Patel said. “The C group is marked by chromosomal instability, meaning these tumors have a higher tendency to add or lose entire chromosomes or sections of them, which affects the gene expression profile.”
The new approach at classifying meningiomas has important implications for patients.
“At the moment, the standard of care is to remove the tumor and counsel the patient. Identifying these groups provides patients with better prognostication,” Patel said.
This work also opens the opportunity to study what makes aggressive meningiomas aggressive, which can lead to future therapies.
Our study and those from other groups over the last few years have set the stage for a molecular classification of meningiomas. For other tumors, gliomas and medulloblastomas, molecular classification has replaced the traditional histopathological classification. We hope that in the next few years it will become standard to use molecular data to classify meningiomas, too,” Patel said.
Read the complete study in the journal Science Advances.
Other contributors to this work include Caroline C. Hadley, Arif O. Harmanci, Akdes S. Harmanci and Tiemo J. Klisch. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine; Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital and the University of Texas Health Science Center, Houston.
This work was supported by the NINDS (grants K08NS102474 R25NS070694), the Roderick D. MacDonald Fund, the Hamill Foundation and P30 Cancer Center Support Grant NCI-CA125123.