Novel marker identifies cells that generate new neurons throughout life
The mammalian center for learning and memory, known as the hippocampus, has a remarkable capacity to generate new neurons throughout life. Newborn neurons originate from neural stem cells (NSCs) and are crucial for forming neural circuits required for learning and memory, and mood control.
“During aging, the number of NSCs declines, leading to decreased neurogenesis and age-associated cognitive decline, anxiety and depression,” said Dr. Mirjana Maletić-Savatić, associate professor of pediatrics-neurology at Baylor College of Medicine and investigator at the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital. “Identifying the core molecular machinery responsible for NSC preservation is of fundamental importance if we are to use neurogenesis to halt or reverse hippocampal age-related conditions.”

One of the major hurdles in exploring this fundamental biological process in the human brain is the lack of specific NSCs markers amenable for advanced imaging and in vivo or live analysis.
“We do not have the tools to study this process in living humans,” said Maletić-Savatić. “All the knowledge we have gathered so far comes from analyses of the postmortem brains and animal model studies.”
Existing NSC markers are present within cells and cannot be reached for visualization in living tissues. Therefore, in collaboration with colleagues from New York and Spain, Maletić-Savatić and Dr. Louis Manganas, associate professor at the Stony Brook University, conducted the current study to find such cell surface markers. They also developed tools, such as molecules that bind to the markers that could be used for positron emission tomography (PET) to visualize them using advanced real-time live brain imaging.
A needle in a haystack
The team looked to identify novel cell surface markers of NSCs among all the proteins that are present on the surface of these cells. For this, they generated antibodies for the surface proteins, which made their mission rather challenging.
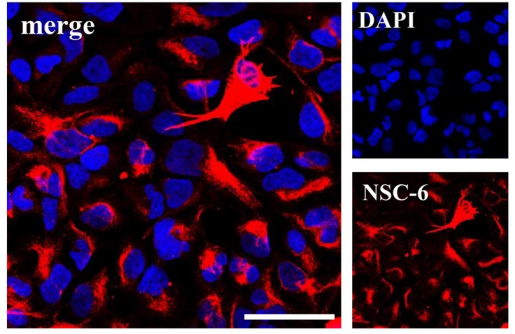
They solved this problem by relying on an age-old method of generating antibodies, which consists on injecting mice with whole-cell or membrane preparations. This resulted in 1648 antibodies, out of which 39 reacted with NSCs. One of the 39 antibodies, called NSC-6, most strongly labeled NSCs.
Mass spectrometric analysis of the human hippocampal tissue revealed that antibody NSC-6 recognized the surface protein Brain-Abundant Signal Protein 1 (BASP-1), which had been previously found to be present in the neurons of the mouse brain but not in NSCs. Interestingly, the NCS-6 antibody that recognizes BASP-1 in NSCs did not label neurons or any other cells apart from NSCs, indicating that it could be used to visualize these cells in the live mammalian brain.
Using our new antibody, we found that BASP-1 is restricted to NSCs in neurogenic niches in mammalian brains, including humans, during development in utero and after birth.”
“Thus, our work identified membrane-bound BASP-1 protein as a possible biomarker of NSCs that would allow us to examine the mechanisms of adult human neurogenesis as well as to explore its role in the process,” said Maletić-Savatić, a member of Baylor’s Dan L Duncan Comprehensive Cancer Center. “The ultimate goal of our research is to maintain neurogenesis throughout life at the same level as it is in the young brain, to prevent the decline in our cognitive capabilities and reduce the tendency towards mood disorders such as depression, as we age.”
With this newly discovered biomarker, scientists can better understand the relevance and intricate mechanisms of neurogenesis, which may lead to new future therapeutic approaches to treat and manage neurological and neuropsychiatric disorders associated with diminished neurogenesis.
Read the complete study in the journal Nature Scientific Reports.
Other authors involved in the study include Irene Durá, Sivan Osenberg, Fatih Semerci, Mehmet Tosun, Rachana Mishra, Luke Parkitny and Juan M. Encinas. They are affiliated with one or more of the following institutions: Baylor College of Medicine, Texas Children’s Hospital, Jan and Dan Duncan Neurological Research Institute, Achucarro Basque Center for Neuroscience, Stony Brook University Medical Center and the Basque Foundation for Science.
The study was funded by grants from the National Institutes of Health, U.S. Army Medical Research, Cynthia and Antony Petrello Endowment, Mark A. Wallace Endowment, the National Institute of Diabetes and Digestive and Kidney Diseases, MINECO, FPI MICINN predoctoral fellowship, the Proteomics Center at Stony Brook University. and the BCM IDDRC.