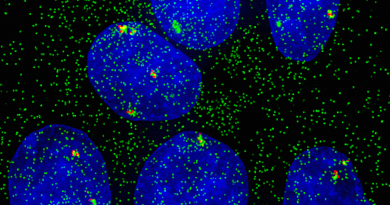
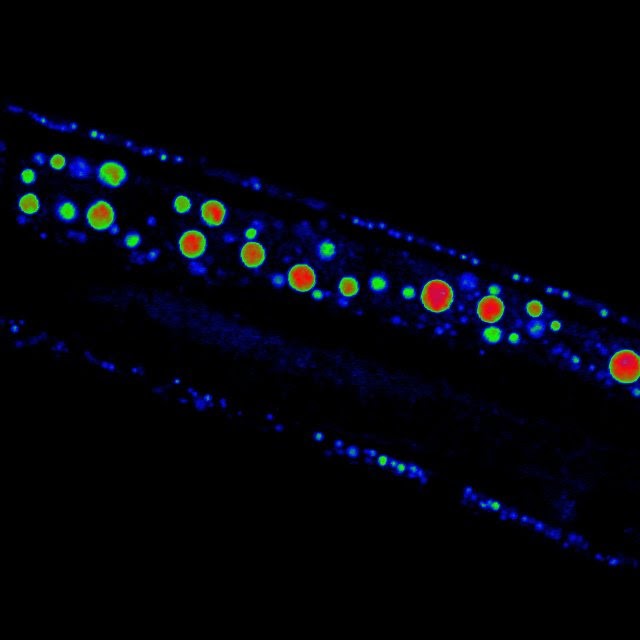
Extending the body’s expiration date may involve the ends of the chromosomes
Scientists may have found a strategy to achieve the science-fictional greeting “Live long and prosper.”
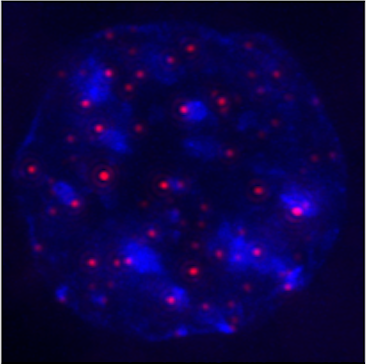
In his lab at Baylor College of Medicine, Dr. Ergun Sahin and his colleagues investigate how to slow down the body’s decline and treat age-related disease by studying the role the ends of the chromosomes or telomeres play in these processes.
“Our genetic material is tightly packed into thread-like structures called chromosomes and at the ends of the chromosomes are particular pieces of DNA called telomeres. Telomeres work like the plastic tips at the end of shoelaces; they prevent chromosomes from unraveling or sticking to each other,” said Sahin, assistant professor in the Huffington Center on Aging and the Department of Molecular Physiology and Biophysics at Baylor.

Telomeres also are involved in aging and disease; as an organism gets older, telomeres shorten and cells progressively deteriorate, stop dividing and die. Telomere shortening during human aging is believed to be a major underlying cause of age-related decline of stem cells – cells with the potential to develop into many different types of cells and help in the healing process in the body.
Telomere shortening also affects the susceptibility of tissues to disease; however, how telomere shortening impairs regeneration and increases risk of disease is not well understood. Evidence suggests that stabilizing telomeres could prevent or slow down aging and disease. In this study, Sahin and his colleagues investigated the effect of restoring telomere length in a mouse model of liver tissue fibrosis.
Manipulating telomere length may help treat age-related disease
Telomere shortening has been associated with increased risk of organ failure and tissue fibrosis, usually in liver and lung, as cells with compromised telomeres fail to divide to replace dying cells.
Previous studies have shown that both telomeres and sirtuins contribute to aging and tissue fibrosis and seem to interact with each other. In this study, Sahin and his colleagues investigated the molecular mechanisms that connected telomeres and sirtuins. For this, they developed a mouse model of liver disease in which the animals were genetically engineered to develop shorter, dysfunctional telomeres and age prematurely. When exposed to certain liver toxins, these animals quickly develop liver fibrosis – scarring of the liver that over time can lead to cirrhosis.
“In these mice, we discovered that shorter telomeres triggered a reduction in the production of sirtuins in liver and other tissues as well,” Sahin said. “This, together with other observations, indicates that telomeres regulate sirtuins.”
Interestingly, the researchers also found that in turn, sirtuins can affect telomeres. When Sahin and his colleagues increased the activity of sirtuins by feeding mice a small molecule – nicotinamide mononucleotide, or NMN, an NAD+ precursor – telomeres were stabilized.
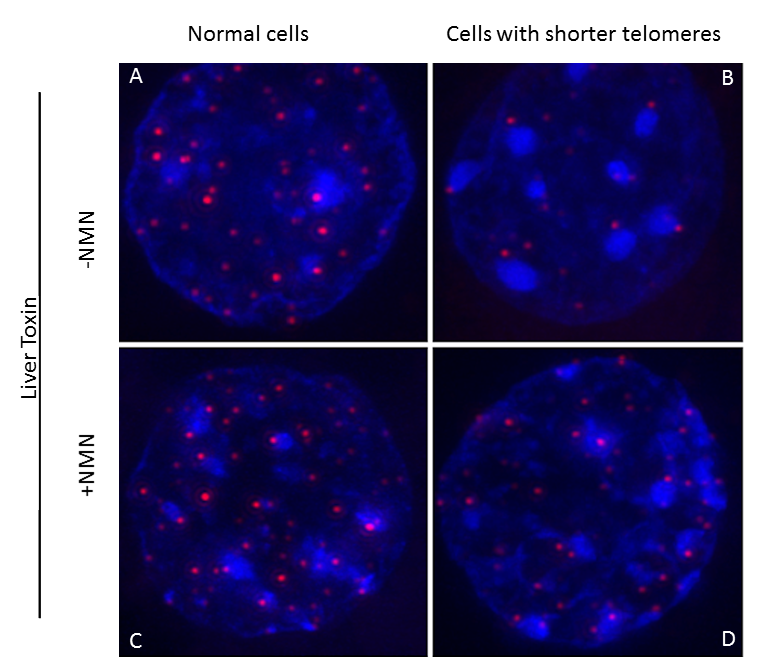
“Furthermore, feeding NAD+ precursor to the mice not only maintained telomere length but also improved liver condition in these mice,” Sahin said.
More research is needed before these findings can be translated into treatments for human conditions.
“It’s important to keep in mind that telomere length can also affect cancer growth. Having shorter telomeres would set cancer cells on a path to self-destruction, but keeping their telomeres long would likely allow them to continue proliferating,” Sahin said. “We plan to continue our investigation on the molecular mechanisms involved in the telomere-sirtuin interactions in order to better understand the benefits as well as the potential risks of telomere length manipulation in health and disease.”
Interested in reading all the details of this work? Find them in the journal Cell Metabolism.
Other contributors to this study include Hisayuki Amano, Arindam Chaudhury, Cristian Rodríguez-Aguayo, Lan Lu, Viktor Akhanov, Andre Catic, Yury V. Popov, Eric Verdin, Hannah Johnson, Fabio Stossi, David A. Sinclair, Eiko Nakamaru-Ogiso, Gabriel López-Berestein, Jeffrey T. Chang, Joel R. Neilson, Alan Meeker, Milton Finegold and Joseph A. Baur. For the list of author affiliations and financial support for this work, visit this link.