Why gut bacteria might be in a recipe for longer, healthier life
The scientific community is increasingly aware that an organism’s interactions with the millions of microbes in their bodies – the microbiome – can influence many of its functions, such as cognitive and metabolic activities and aging. Questions have also been raised about the role the genetic composition of the microbiome might be playing in longevity. In her lab at Baylor College of Medicine, Dr. Meng Wang, associate professor of molecular and human genetics and the Huffington Center On Aging, investigated this possibility.

“This question is difficult to explore in mammals due to technical challenges, so we turned to the laboratory worm C. elegans,” said Wang.

C. elegans is a transparent, simple organism that is as long as a pinhead and shares essential characteristics with human biology. During its 2 to 3 week long lifespan, the worm feeds on bacteria, develops into an adult, reproduces, and progressively ages, loses strength and health and dies. Many research laboratories around the world, including the Wang lab, work with C. elegans to learn about basic biological processes.
“We think that C. elegans is a wonderful system in which to study the connection between bacterial genes and aging because we can very fine tune the genetics of microbes and test many genes in the worm in a relatively short time,” Wang said.
Testing thousands of genes, one at a time.

To study the effect of individual bacterial genes on the lifespan of C. elegans, Wang joined efforts with Dr. Christophe Herman, associate professor of molecular and human genetics and molecular virology and microbiology at Baylor, and other colleagues who are experts in bacterial genetics. They employed a complete gene-deletion library of bacterium E. coli; a collection of E. coli, each lacking one of close to 4,000 genes.
“We fed C. elegans each individual mutant bacteria and then looked at the worms’ life span,” Wang said. “Of the nearly 4,000 bacterial genes we tested, 29, when deleted, increased the worms’ lifespan. Twelve of these bacterial mutants also protected the worms from tumor growth and accumulation of amyloid-beta, a characteristic of Alzheimer’s disease in humans.”
Further experiments showed that some of the bacterial mutants increased longevity by acting on some of the worm’s processes known to be linked to aging. Other mutants encouraged longevity by over-producing the polysaccharide colanic acid. When the scientists provided purified colanic acid to C. elegans, the worms also lived longer. Colanic acid also showed similar effects in the laboratory fruit fly and in mammalian cells cultured in the lab.
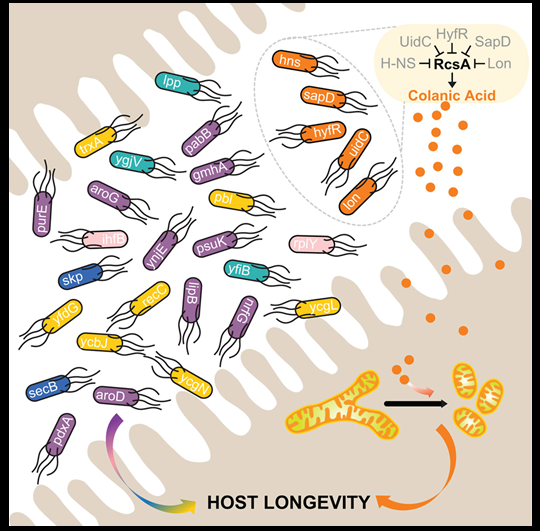
The researchers propose that, based on these results, it might be possible in the future to design preparations of bacteria or their compounds that could help slow down the aging process.
Colanic acid mediates crosstalk between bacteria and mitochondria
Interestingly, the scientists found that colanic acid regulates the fusion-fission dynamics of mitochondria, the structures that provide the energy for the cell’s functions.
“These findings are also interesting and have implications from the biological point of view in the way we understand host-microbe communication,” Wang said.
“Mitochondria seem to have evolved from bacteria that millions of years ago entered primitive cells. Our finding suggests that products from bacteria today can still chime in the communication between mitochondria in our cells. We think that this type of communication is very important and here we have provided the first evidence of this. Fully understanding microbe-mitochondria communication can help us understand at a deeper level the interactions between microbes and their hosts,” said Wang.
Read all the details about this study in Cell.
Other contributors to this work include Bing Han, Priya Sivaramakrishnan, Chih-Chun J. Lin, Isaiah A.A. Neve, Jingquan He, Li Wei Rachel Tay, Jessica N. Sowa, Antons Sizovs, Guangwei Du and Jin Wang.
Financial support for this project was provided by the National Institutes of Health grants R01AG045183, R01AT009050, DP1DK113644, R01HL119478, R01GM088653, R01GM115622, R01CA207701 and Howard Hughes Medical Institute Faculty Scholar Award.