What activates the PI3K pathway, the most commonly mutated pathway in cancer that can be targeted by drugs?
The PI3K pathway has a number of diverse functions, including altering the cell’s metabolism and driving cell growth and proliferation. The PI3K pathway also is the most commonly mutated pathway in cancer that can be targeted by drugs.
“Our multi-institutional research team studied the PI3K pathway, one of the most important pathways of the cell,” said Dr. Chad Creighton, associate professor of medicine and member of the Dan L Duncan Comprehensive Cancer Center Division of Biostatistics at Baylor College of Medicine. “We took a comprehensive approach to evaluating which molecular changes in cancer cells are most likely involved with PI3K in the development of the disease.”
Previous studies had identified a number of the genes, proteins and processes involved in the PI3K pathway in cells grown in the lab.

“In this study, we have taken what we have learned in the lab regarding how the pathway works and analyzed it together with information about the genes and the proteins present in cancer cells taken from human patients,” Creighton said.
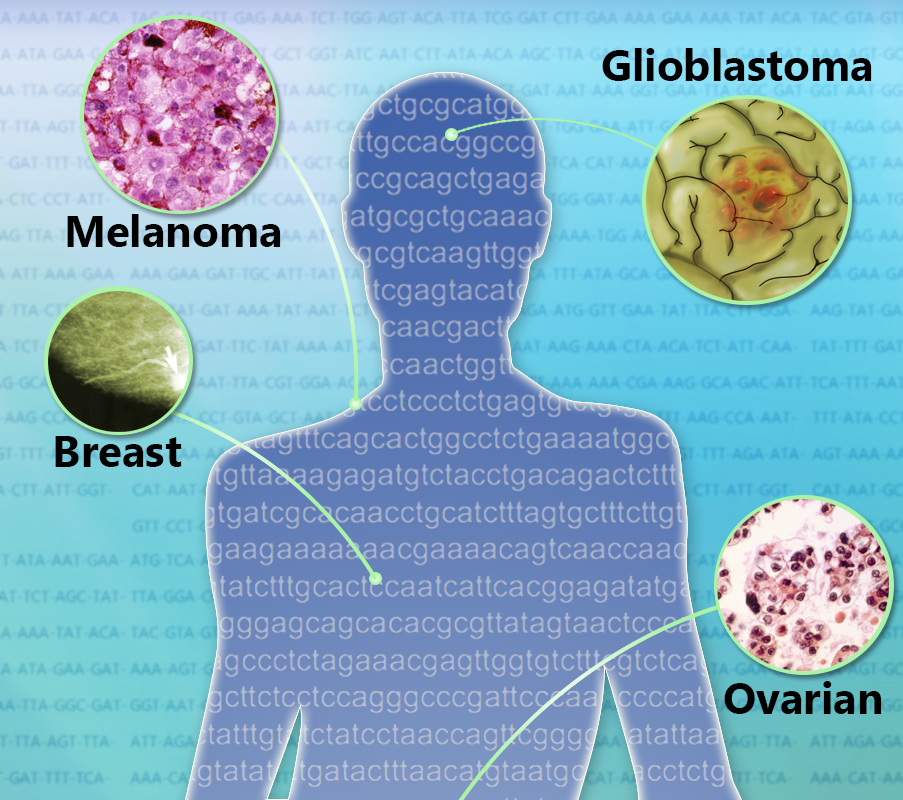
“We looked at nearly 11,000 human cancers representing 32 major types.”
“This is the largest study of its kind, and it was possible in part thanks to the Cancer Genome Atlas, a publicly available dataset of genomic changes in 32 types of cancer.”
To carry out the complex analysis of this vast amount of data, the research team pulled the resources of experts in cancer protein data, in molecular biology of the pathway, and in the use of powerful analytical tools that provided genomic analysis and integration of the protein data.
The challenge is to know which mutations in cancer are important
To assess which cancer mutations are important, the researchers carried out a comprehensive analysis that allowed them to distinguish which of the altered genes and proteins were more likely to affect the normal function of the PI3K pathway.
“What makes this analysis complex is that there is a large number of genes and protein alterations that can be present in a given patient’s tumor, and it is possible that different alterations are present in different patients,” Creighton said. “In addition, not all mutations necessarily cause disease.”
“The challenge is to find out which mutations are altering the pathway in a way that can lead to cancer. We hope that one day we will be able to apply this knowledge to personalized medicine.”
There were a few surprises in the study.
“For some genes there was previous work indicating they were implicated in this pathway, but we discovered other genes, such as IDH1 and VHL, which had not been typically associated with the pathway in cancer before,” Creighton said. “These genes, as well as others that may be discovered in the future, may now be incorporated into the group of genes linked to the PI3K pathway and considered as potential candidates for targeted therapy.”
“Finding several cancers and mutations that we didn’t know before could activate this pathway supports moving up the priority of testing drugs toward the new mutations found in specific cancer types,” said co-author Dr. David Kwiatkowski, professor of medicine at Harvard Medical School and senior physician at Brigham and Women’s Hospital and the Dana Farber Cancer Institute.

The future of personalized medicine
“The comprehensive nature of this project that integrates information from multiple levels has the potential to impact patient management and to eventually improve outcomes for the large population of patients with abnormalities in this very important pathway,” said Dr. Gordon Mills, professor of medicine and immunology at MD Anderson Cancer Center.
“This comprehensive approach expands our knowledge regarding which types of cancer this pathway is activated and why, and that’s important in terms of thinking about therapies that go after this pathway,” Kwiatkowski said.
Imagine the following possible future scenario in a personalized medicine setting: a patient provides a sample of tumor and the physician sends it to a lab that runs a sequencing assay that shows where the genetic changes are located and the type of changes. Then, from the protein data, the team of physicians and scientists can determine which genetic changes are associated with greater activation of the PI3K pathway and which may not. These data would help the team in terms of selecting patients for whom specific drugs may be effective.
Read all the details about this study, and the complete list of authors and their affiliations in Cancer Cell.
This work was supported in part by the Cancer Prevention and Research Institute of Texas grant RP120713 C2, the National Institutes of Health grants 5P50CA098258, 5U01CA168394, U24CA143883, 2P50CA101942-11A1, 1R21CA191687, P01CA120964, CA175486, CA209851 and P30CA125123, and the Cancer Center Support Grant 30CA016672.