Rap1, a potential new target to treat obesity
Understanding how the body keeps a healthy weight is like solving a puzzle with pieces still missing. Some of the unknown pieces are genes located in the brain and scientists are interested in unveiling which these genes are and how their functions are affected by their interaction with diet, high-fat diet in particular.
The gene Rap1 is a new piece of the puzzle. Rap 1 is expressed in a variety of tissues, including the brain where it is involved in functions such as memory and learning. Little was known, however, of the role brain Rap1 plays in energy balance.
Scientists at Baylor College of Medicine, the National Institutes of Health and Virginia Tech Carilion Research Institute have discovered that Rap1 is involved in a new mechanism in the mouse brain that regulates obesity. The study, which appears in Cell Reports today, shows that this new mechanism can potentially be targeted to treat obesity.

“It’s well known that the brain is involved in the development of obesity, but how a high-fat diet changes the brain so it triggers the accumulation of body fat is still unclear,” said senior author Dr. Makoto Fukuda, assistant professor of pediatrics at Baylor and the USDA/ARS Children’s Nutrition Research Center at Baylor and Texas Children’s Hospital.
Better understanding how Rap1 works
To explore the role Rap1 plays in a mouse model, the scientists selectively deleted the Rap1 gene in a group of neurons in the hypothalamus, a region of the brain that is involved in regulating whole-body metabolism.
Fukuda and colleagues had two groups of mice. In one group, the mice were genetically engineered to lack the Rap1 gene, while the control group had a functional Rap 1 gene. Then, the scientists fed the mice in both groups a high-fat diet in which 60 percent of the calories came from fat. As expected, the control mice with a working Rap1 gene gained weight, but the mice that lacked Rap 1 did not. Interestingly, when both groups of mice were fed a normal diet, both showed similar weights and body fat.
The scientists then looked closer at why the mice lacking the Rap1 gene had not gained weight despite eating a high-fat diet.
“We observed that the mice lacking Rap1 were not more physically active. However, they ate less and burned more body fat than mice with Rap1,” said Fukuda.
“These observations were associated with the hypothalamus producing more of a hormone that reduces appetite, called POMC, and less of hormones that stimulate appetite, called NPY and AgRP.” These mice also had lower levels of blood glucose and insulin than controls.
The scientists also were interested in studying whether leptin changed in mice lacking Rap1. Leptin, the ‘satiety hormone’ produced by fatty tissue, helps regulate body weight by inhibiting appetite. Obese people, however, do not respond to leptin’s signals of satiety, and the blood levels of leptin are higher than those in non-obese people. Leptin resistance is a hallmark of human obesity.
Mice that lacked Rap1 and ate a high-fat diet, on the other hand, did not develop leptin resistance; they were able to respond to leptin, and this was reflected in the hormone’s lower blood levels.
Fukuda and colleagues also tested the effect of inhibiting Rap1 with drugs instead of deleting the gene on mice on a high-fat diet. The scientists inhibited RAP1 action with inhibitor ESI-05.
“When we administered ESI-05 to obese mice, we restored their sensitivity to leptin to a level similar to that in mice eating a normal diet. The mice ate less and lost weight,” said Fukuda.
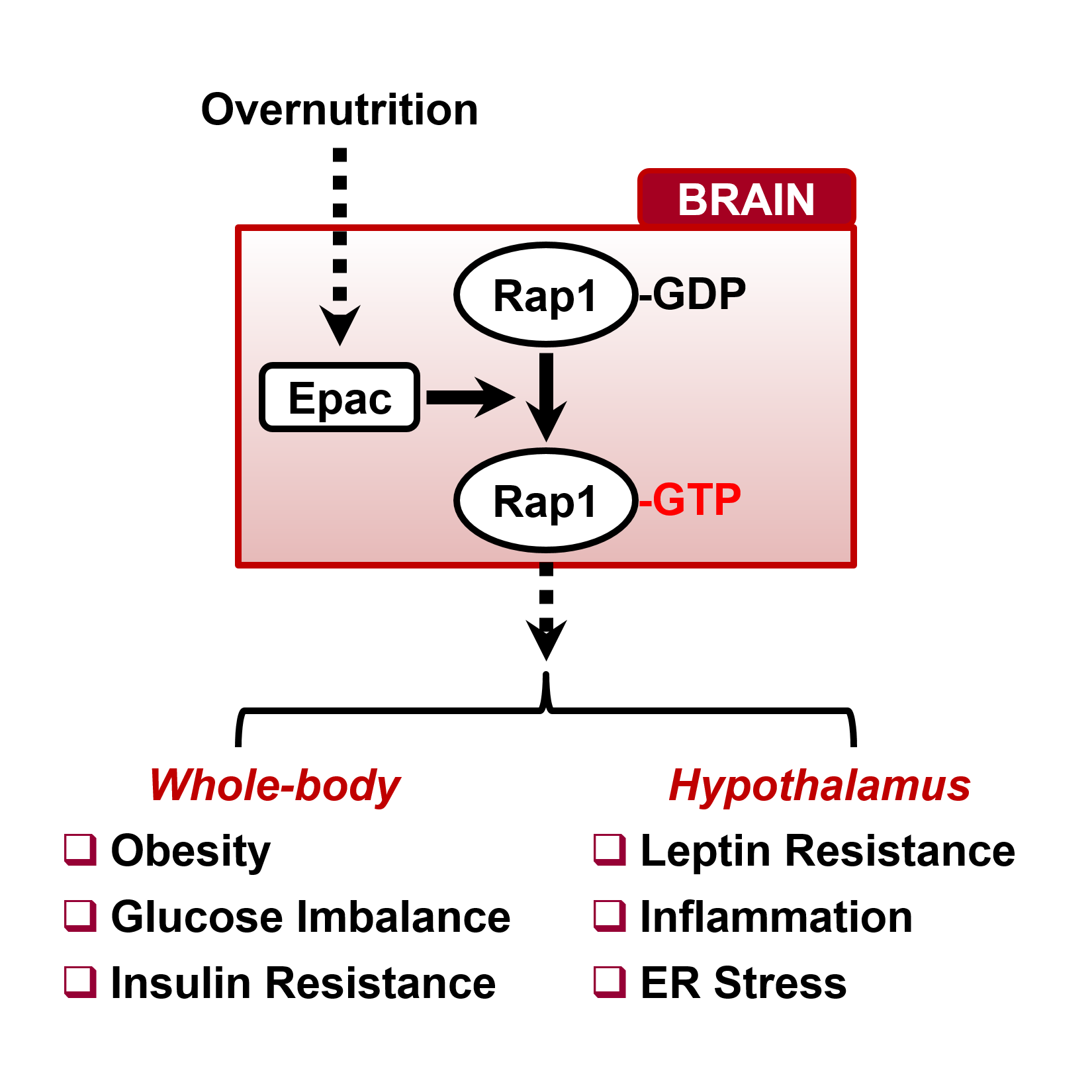
“This new mechanism involving Rap1 in the brain may represent a potential therapeutic target for treating human obesity in the future,” said Fukuda.
###
Other contributors to this work include Kentaro Kaneko, Pingwen Xu, Elizabeth L. Cordonier, Siyu S. Chen, Amy Ng, Yong Xu and Alexei Morozov.
This work was supported by USDA CRIS 6250-504 51000-055, AHA-14BGIA20460080, NIH-P30-DK079638 and NIH R01DK104901, AHA-505 15POST22500012 and the Uehara Memorial Foundation 201340214.