Genomic study reveals immune factors contributing to pediatric heart transplant failure

Pediatric heart transplantation has long been hailed as a life-saving intervention for children suffering from end-stage heart failure. While the procedure offers hope, the long-term outcomes for these young patients remain suboptimal due to transplant rejection and graft failure.
In a groundbreaking study published in the journal Circulation, researchers from The Texas Heart Institute, Baylor College of Medicine, Texas Children’s Hospital and the University of Texas Health Science Center McGovern Medical School have shed light on the cellular landscape within transplanted pediatric hearts, paving the way for improved treatment strategies and enhancing the longevity of heart transplants.

The research, led by physician-scientist at The Texas Heart Institute (THI), Dr. James F. Martin, Vivian L. Smith Chair in Regenerative Medicine and vice chairman and professor of integrative physiology at Baylor, single-cell genomics expert Dr. Xiao Li, THI faculty and assistant investigator of the McGill Gene Editing Lab at THI and Dr. Diwakar Turaga, assistant professor of pediatrics – critical care at Baylor and pediatric cardiac intensivist at Texas Children’s Hospital, utilized a unique dataset comprising rare heart samples from repeat heart transplantations. By harnessing cutting-edge single-nucleus RNA sequencing (snRNA-seq) techniques, the researchers could delve deep into the inflammatory heart microenvironment within human pediatric cardiac transplants.
“Our approach offers an unprecedented level of detail,” said Martin. “We were able to distinguish immune cells originating from the donor versus the recipient by leveraging naturally occurring genetic variants embedded within our sequencing data. This helps us gain a comprehensive understanding of the immune response dynamics within transplanted hearts.”
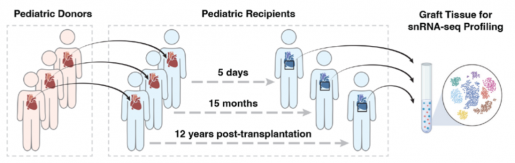
The study, which marks the first-ever description of the immune cells within a transplanted pediatric heart at single-cell resolution, examined samples collected as early as five days post-transplantation and extending up to 12 years thereafter.
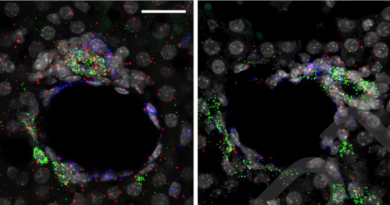
Through meticulous analysis, the researchers discovered a rapid loss of donor-derived tissue-resident macrophages, cells that are crucial for graft acceptance and long-term success. In contrast, macrophages derived from the recipient’s circulation swiftly populated the heart shortly after transplantation. This imbalance between donor-derived and recipient-derived macrophages significantly contributed to transplant failure.

“These findings have significant clinical implications,” explained Li. “By targeting the heightened inflammatory response mediated by recipient-derived macrophages and natural killer cells, we can potentially prevent early graft failure and acute rejection episodes. Additionally, preserving the population of resident macrophages within the transplanted heart could pave the way for novel immunomodulation strategies and greatly enhance the longevity of pediatric cardiac transplants.”

“In the Cardiac Intensive Care Unit (CICU), I take care of children who come in with heart rejection. Our medical therapies to treat rejection are still very limited. This study is a major step towards targeted immune therapies and precision medicine,” Turaga said.
Together, the team’s collective efforts have advanced our understanding of immune response dynamics in transplanted pediatric hearts that contribute to the organ’s rejection and failure. With further research and clinical implementation, these insights hold the potential to transform the lives of young patients and improve their long-term quality and quantity of life.
Rich G. Li, Chang-Ru Tsai, Julianna N. Quinn, Yi Zhao, Ruby Wilson, Katherine Carlson, Jun Wang, Joseph A. Spinner, Edward J. Hickey, Iki Adachi also contributed to this work.
This study was supported by the Don McGill Gene Editing Laboratory of The Texas Heart Institute, the American Heart Association (824138, 970606 and 23EIA1039128) and National Institutes of Health (R01HL142704).
Read more here.
Follow From the Labs on X @BCMFromtheLabs and Instagram!