Human mini guts reveal new insights into Cronkhite-Canada syndrome and potential new therapies
Researchers at Baylor College of Medicine and collaborating institutions working with human intestinal organoids, also called mini guts, have shed new light on the potential causes of Cronkhite-Canada syndrome, a rare condition characterized by abundant non-cancerous growths or polyps in the intestine and other symptoms such as hair and nail loss and changes in skin pigmentation.
“During my clinical rounds, I met a patient with Cronkhite-Canada syndrome and became very interested in better understanding this little known condition,” said first author Victoria Poplaski, a graduate student in Dr. Sarah Blutt’s lab at Baylor. “Patients with Cronkhite-Canada syndrome have diarrhea, weight loss, abdominal pain, anorexia and nausea. About 500 people have been diagnosed with this disease worldwide so there have not been many opportunities to investigate it to identify its causes and potential therapies.”

Poplaski and her colleagues applied their expertise in developing human intestinal organoids to take a closer look at what could be triggering so much proliferation in the patient’s intestine. Human intestinal organoids, or mini guts grown in the lab, model human intestinal cells, closely representing actual small intestine tissue and its functions.
Mini guts reveal a new role for serotonin in intestinal cell proliferation
Poplaski received a biopsy sample or tissue from the patient’s intestine and developed mini guts from it in the lab.
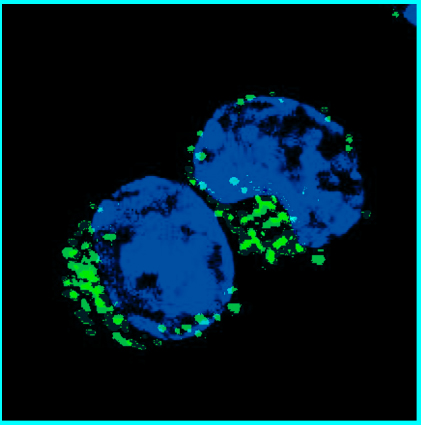
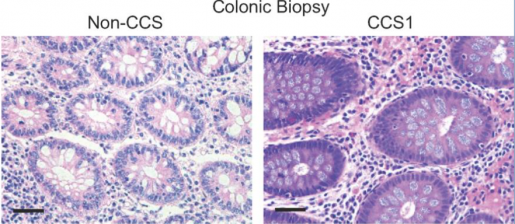
We discovered that, compared to mini guts from people without Cronkhite-Canada syndrome, the patient-derived mini guts were highly proliferative. They also had many more enteroendocrine cells, the cell type in the intestine that specializes in producing hormones, and the enteroendocrine cells abundantly present in the patient’s mini guts produced serotonin,” Poplaski said.
“We confirmed these features in patient tissue biopsies.”
More than 90% of serotonin in the body is produced by the enteroendocrine cells in the intestinal tract. Although serotonin is best known for its role as a neurotransmitter in the central nervous system, it also affects a variety of functions in other organs, including some tasks of the intestinal epithelium.

“Supporting the novel role of serotonin in intestinal epithelial proliferation, we found that treating non-Cronkhite-Canada syndrome organoids with serotonin increased their proliferation and inhibiting serotonin production in the Cronkhite-Canada syndrome organoids decreased proliferation, suggesting a link between local serotonin production and control of epithelial intestinal cell proliferation,” said Blutt, associate professor of molecular virology and microbiology at Baylor and corresponding author of the work.
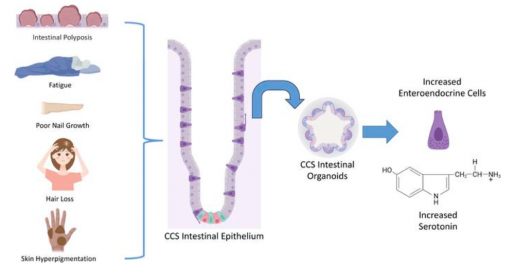
The findings suggest the potential benefit of serotonin inhibitors to treat this condition.”
This research is a perfect example of how organoid cultures can lead to new discoveries about the biology underlying rare diseases.

“When we started this research, some people said it was not worth it because initially we only had a single patient to study. I disagreed and thought we might find something worthwhile,” said co-author Dr. Mary Estes, Distinguished Service Professor of Molecular Virology and Microbiology and Cullen Foundation Endowed Chair at Baylor. She also is a member of Baylor’s Dan L Duncan Comprehensive Cancer Center. “The results from the organoids derived from that single patient yielded completely unexpected and exciting data that might lead to new therapies. Then we were able to confirm these results with organoids from a second patient. I am hoping this study will lead to contacts with more physicians caring for patients with Cronkhite-Canada syndrome and a clinical trial to test the new basis for a process that leads to this condition that we discovered in this work.”
Find all the details of this work in The Journal of Clinical Investigation and a short video presenting this research, here.
Other contributors to this work include Carolyn Bomidi, Amal Kambal, Hoa Nguyen-Phuc, Sara C. Di Rienzi, Heather A. Danhof, Xi-Lei Zeng, Linda A. Feagins, Nan Deng, Eduardo Vilar, Florencia McAllister, Cristian Coarfa, Soyoun Min, Hyun Jung Kim, Richa Shukla and Robert Britton. The authors are affiliated with one of the following institutions: Baylor College of Medicine, the University of Texas at Austin Dell Medical School, the University of Texas MD Anderson Cancer Center-Houston or the Lerner Research Institute at Cleveland Clinic.
See the publication for a complete list of the financial support for this project.
Follow From the Labs on Twitter @BCMFromtheLabs.