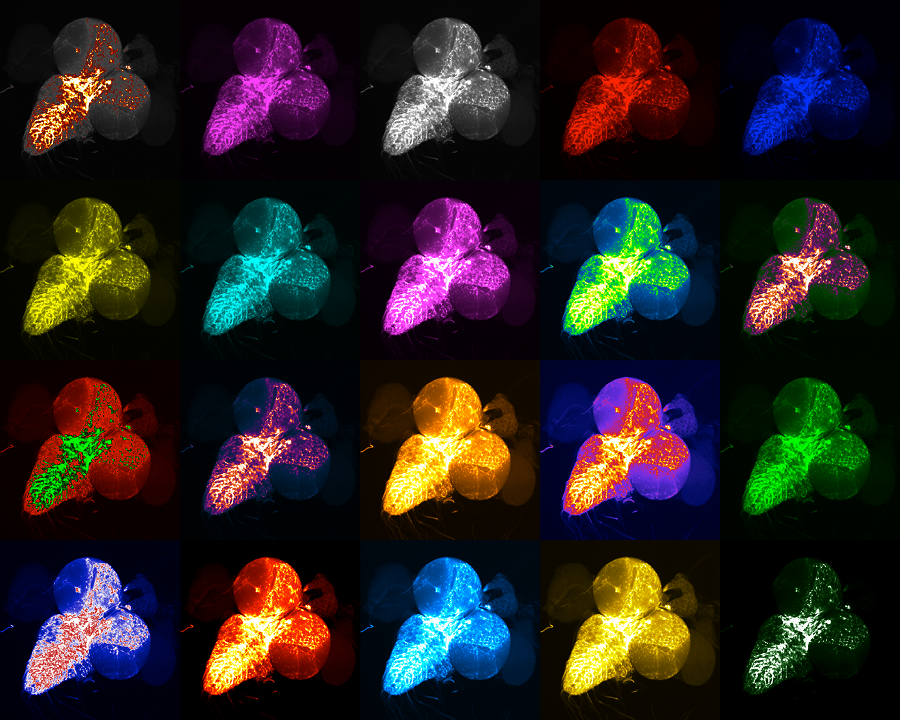
Neurons lead the way in the development of astrocytes
Astrocytes play diverse roles that are vital for proper brain function. For instance, they support the activity of other essential brain cells, neurons; participate in the formation and function of synapses, essential neuron-to-neuron connections; release neurotransmitters, chemicals that mediate neuronal communication; and make the blood-brain barrier.
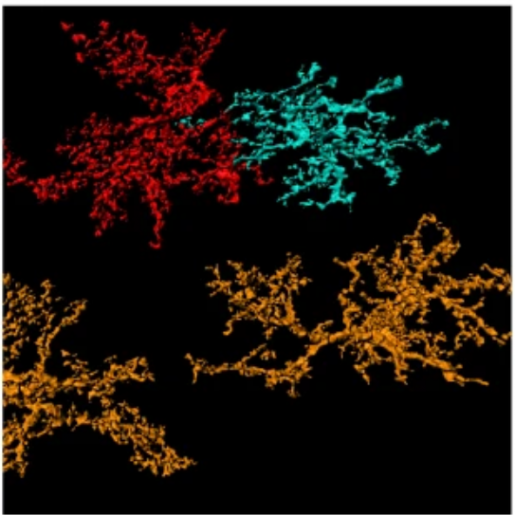
“In the adult brain, the bushy shape of astrocytes is fundamentally linked to effective brain function. The ends of the branched-out astrocyte structure interact with neurons and regulate synaptic activity,” said first author Yi-Ting Cheng, a graduate student in Dr. Benjamin Deneen’s lab at Baylor . “If astrocytes lose their structure, then synapses do not behave properly and brain function goes awry.”

Figuring out how astrocytes acquire their complex, bushy structure is essential to understanding how the brain develops and functions and may bring new insight into how neurodevelopmental conditions emerge. In this study, published in the journal Nature, the researchers investigated the cells and processes that direct the development of astrocyte structure.
Neurons lead the way
When astrocytes develop, neurons are already present and active, so do neurons influence how astrocytes acquire their complex shape?
“We artificially activated or silenced neurons and determined whether this would speed up or slow down astrocyte maturation,” Cheng said.
We found that neuronal activity is both necessary and sufficient to drive full astrocyte maturation into a bushy-shaped cell.”
So how are astrocytes receiving the signals that direct them down to the proper maturation path? Through several experimental approaches the team discovered that neurons produce a neurotransmitter called GABA that binds to astrocytes via a molecule on their surface named GABAB receptor.

“We knocked out the GABAB receptor in astrocytes and activated the neurons. In this situation, the neurons did not promote the development of a typical astrocyte shape, supporting the idea that neurons communicate with astrocytes via the GABAB receptor to promote their maturation,” said Deneen, professor and Dr. Russell J. and Marian K. Blattner Chair in the Department of Neurosurgery and director of the Center for Cancer Neuroscience at Baylor. He also is an investigator at the Jan and Dan Duncan Neurological Research Institute and the corresponding author of the work.
“This finding was surprising and very interesting,” Deneen said. “Neurotransmitters such as GABA are known to signal between neurons at synapses, but we discovered that neurotransmitters also signal astrocytes, influencing their development by triggering changes in their structure.”
Other experiments revealed more pieces of the puzzle of how neurons lead astrocytes to develop their bushy shape. “Neurons produce GABA, which binds to astrocytes via the GABAB receptor. In turn, this activates a series of events, including triggering the expression of another receptor called Ednrb, which drives pathways that remodel cellular architecture inside the cells associated with cell shape,” Cheng said.
The researchers also investigated another mystery related to astrocyte development. They found that regulation of the expression of GABAB receptor in astrocytes does not occur in the same way in different brain regions. “This result was totally unexpected,” Deneen said. “The GABAB receptor is universally required for astrocytes to develop their bushy shape in all brain regions. How is it regulated differently in different areas of the brain?”
Through bioinformatics analyses the researchers discovered that this regional regulation is conferred by two proteins, LHX2 in the brain cortex and NPAS3 in the olfactory bulb through their region-specific interactions with proteins SOX9 and NFIA, which are present in all astrocytes where they regulate GABAB receptor expression. In the cortex, LHX2 only binds to NFIA, while in the olfactory bulb NPS3 only binds to SOX9, enabling each one to regulate GABAB receptor expression in a specific brain region.
Altogether, the findings suggest that astrocyte development and function involve a complex pattern of events and proteins triggered by the activity of neurons and that operate in a region-specific manner.
Estefania Luna-Figueroa, Junsung Woo, Hsiao-Chi Chen, Zhung-Fu Lee and Akdes Serin Harmanci, all at Baylor College of Medicine, also contributed to this work.
This work was supported by National Institutes of Health (NIH) grants NS071153, AG071687 and NS096096, the David and Eula Wintermann Foundation, NIH shared instrument grants S10OD023469, S10OD025240 and P30EY002520, the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672), the NIH (CA125123 and RR024574) and the NIH Eunice Kennedy Shriver National Institute of Child Health & Human Development under Award Number P50HD103555.