What to expect within the tumor microenvironment
Breast cancer is very heterogeneous. For many years researchers have recognized different subtypes of breast cancer, for instance, estrogen receptor positive (ER+), ER- and triple negative, and these categories can be further divided into subcategories.
Adding to the complexity of the tumor microenvironment, in recent years it has been increasingly appreciated that immune cells contribute to tumor progression and, importantly, to the tumor’s response to therapy.
“To tumor cell heterogeneity, we now have to add the diversity of the immune cell component in the tumor microenvironment,” said Dr. Xiang ‘Shawn’ Zhang, professor at the Lester and Sue Smith Breast Center and member of the Dan L Duncan Comprehensive Cancer Center at Baylor College of Medicine.

One of the interests of the Zhang lab is to better understand the role immune cells within tumors play in tumor growth and in response to therapy. In this study, Zhang and his colleagues conducted a series of analyses to profile the immune cell composition of tumor microenvironments in eight murine models and in clinical datasets of triple negative breast cancers.
A diversity of immune cell profiles and roles
“We focused on two types of immune cells, neutrophils and macrophages, to investigate whether different tumors had the same immune cell composition and whether seemingly similar immune components played the same role in tumor growth. Importantly, we wanted to find out whether differences in immune cell composition contributed to the tumors’ responses to immunotherapy,” said Zhang, a McNair Scholar at Baylor.
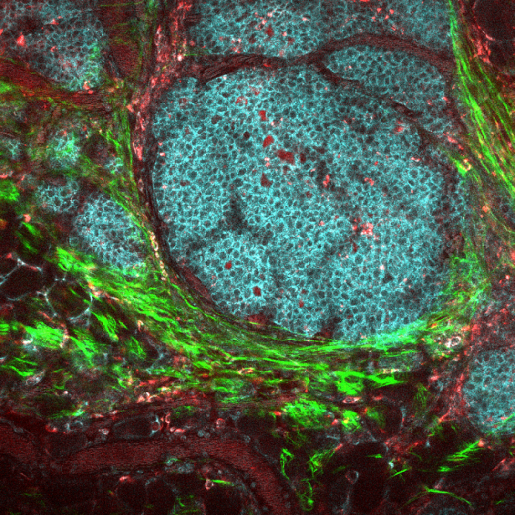
The researchers found large diversity in the frequency of neutrophils and macrophages among the tumor samples, including some tumors that preferentially attracted macrophages, and others that attracted more neutrophils. The predominance of one cell type over the other can be explained in part by the type of molecules produced by the tumor. As Zhang explained, some tumors secrete molecules that attract macrophages, while other tumors produce other molecules that lure neutrophils to the tumor site.
Interestingly, macrophages and neutrophils tended to exclude each other.
“Once one type of cell starts accumulating in the tumor, the other will tend to stay away,” Zhang said. “What supports one type of cell, does not seem to support the other.”
Exploring the roles macrophages and neutrophils play in tumor growth revealed that in some tumors macrophages favored tumor growth, while in others they helped control it. Neutrophils, on the other hand, tended to promote tumor growth.
“These findings are just the beginning. They highlight the need to investigate these two cellular types deeper. Under the name ‘macrophages’ there are many different cellular subtypes and the same stands for neutrophils,” Zhang said.
We need to identify at single cell level which immune cell subtypes favor and which ones disrupt tumor growth taking also into consideration tumor heterogeneity as both are relevant to therapy.”
The report appears in the journal Nature Cell Biology.
Other contributors to this work include first author Ik Sun Kim, Yang Gao, Thomas Welte, Hai Wang, Jun Liu, Mahnaz Janghorban, Kuanwei Sheng, Yichi Niu, Amit Goldstein, Na Zhao, Igor Bado, Hin-Ching Lo, Michael J. Toneff, Tuan Nguyen, Wen Bu, Weiyu Jiang, James Arnold, Franklin Gu, Jian He, Deborah Jebakumar, Kimberly Walker, Yi Li, Qianxing Mo, Thomas F. Westbrook, Chenghang Zong, Arundhati Rao, Arun Sreekumar and Jeffrey M. Rosen. Find the complete list of author affiliations here.
Financial support was provided by the Breast Cancer Research Foundation NCI CA151293 and NCI-CA16303, U.S. Department of Defense DAMD W81XWH-16-1-0073 and W81XWH-18-1-0574, Susan G. Komen CCR14298445 and McNair Medical Institute. The National Institutes of Health (grants P30 AI036211, P30 CA125123 and S10 RR024574) supported the Cytometry and Cell Sorting Core at Baylor College of Medicine.