Timing exercise with muscle fuel switching could help you lose weight
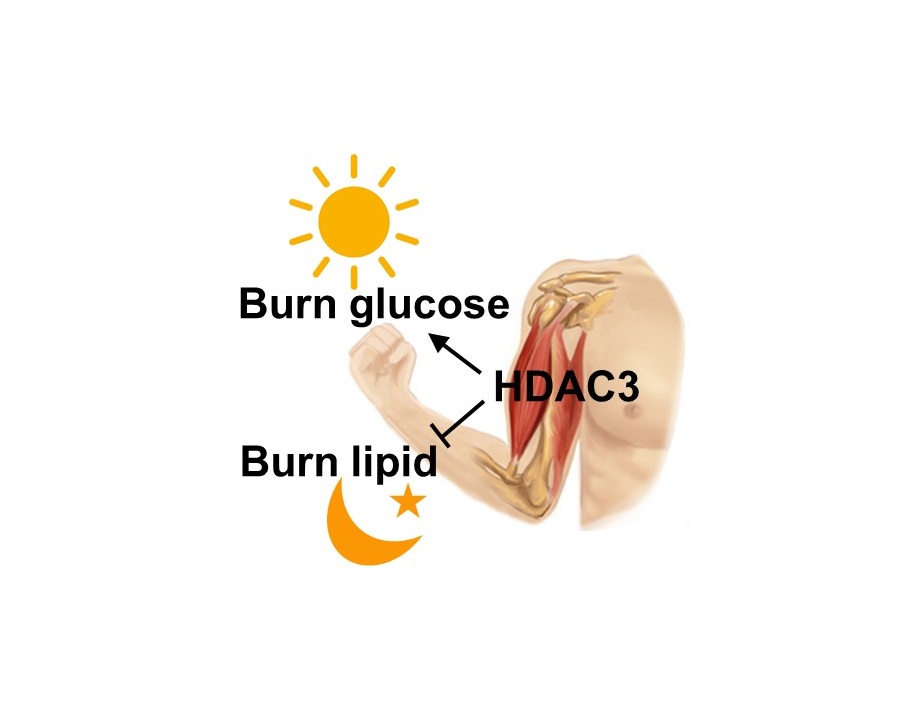
Skeletal muscles change their fuel preference according to a circadian rhythm. Mouse muscles, for instance, use glucose as fuel when the animals are awake and active and switch to fat when they are asleep. Understanding the molecular mechanisms that control the switch from glucose to fat can have significant repercussions in how people manage diabetes, body weight and physical endurance.

“How the muscle uses glucose is regulated by its internal circadian clock that anticipates the level of its activity during the day and at night,” said senior author Dr. Zheng Sun, assistant professor of medicine – diabetes, endocrinology and metabolism, and of molecular and cellular biology at Baylor. “The circadian clock works by turning certain genes on and off as the 24-hour cycle progresses. HDAC3 is a key connection between the circadian clock and gene expression. Our previous work showed that HDAC3 helps the liver alternate between producing glucose and producing lipid. In this work, we studied how HDAC3 controls the use of different fuels in skeletal muscle.”
Skeletal muscles are important in the control of blood glucose in the body. They consume most of the glucose, and if they develop insulin resistance and consequently are not able to use glucose, then diabetes will likely develop. To study the role of HDAC3 in mouse skeletal muscle, Sun and colleagues genetically engineered laboratory mice to deplete HDAC3 only in the skeletal muscles. Then they compared these knocked out mice with normal mice regarding how their muscles burn fuel.
Unexpected results
When normal mice eat, their blood sugar increases and insulin is released, which stimulates muscles to take in and use glucose as fuel. “When the knocked out mice ate, their blood sugar increased and insulin was released as expected, but their muscles refused to take in and use glucose,” said Sun. “Lacking HDAC3 made the mice insulin resistant and more prone to develop diabetes.”
Yet, when the HDAC3-knocked out mice ran on a treadmill, they showed superior endurance, “which was intriguing because diabetes is usually associated with poor muscle performance,” said Sun. “Glucose is the main fuel of muscle, so if a condition limits the use of glucose, the expectation is low performance in endurance exercises. That’s the surprise.”
The researchers then studied what fueled the HDAC3-knocked out mice’s stellar performance using metabolomics approaches and found that the muscles had changed from burning glucose to burning lipids very efficiently. This explains the high endurance, because the body carries a much larger energy reservoir in the form of lipid than carbohydrate. Making muscles burn more fat and less glucose can increase exercise endurance, but could simultaneously cause diabetes.
The finding challenges the widely-used carbohydrate-loading (carbo-loading) strategy for improving endurance performance. “Carbo-loading didn’t make evolutionary sense before the invention of agriculture,” said Sun. “Switching muscles from using carbohydrates to lipids could increase exercise endurance, especially for low-intensity exercise.” The study suggests that HDAC inhibitors, a class of small molecule drugs currently being tested for treating several diseases, could potentially be used to manipulate such fuel switch in muscle and therefore raises concern of doping.
Link to the body’s internal clock
The team performed a number of functional genomics studies that established the link between HDAC3 and the circadian clock. “In normal mice, when the mouse is awake, the clock in the muscle anticipates a feeding cycle and uses HDAC3 to turn off many metabolic genes. This leads the muscles to use more carbohydrate,” said Sun. “When the animal is about to go to sleep and anticipates a fasting cycle, the clock removes HDAC3. This leads the muscles to use more lipid.”
Although these studies were done in mice, the researchers speculate that human muscles most likely will follow the same cycle. The study opens the possibility of promoting body fat burning by increasing exercise activity during the periods in which muscles use lipid, which is at night for people. “Losing body fat would be easier by exercising lightly and fasting at night,” said Sun. “It’s not a bad idea to take a walk after dinner.”
The study appears in Nature Medicine.
Other contributors to this work include Sungguan Hong, Wenjun Zhou, Bin Fang, Wenyun Lu, Emanuele Loro, Manashree Damle, Guolian Ding, Jennifer Jager, Sisi Zhang, Yuxiang Zhang, Dan Feng, Qingwei Chu, Brian D Dill, Henrik Molina, Tejvir S Khurana, Joshua D Rabinowitz and Mitchell A Lazar. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine, University of Pennsylvania, Princeton University, Shanghai Jiao Tong University and Rockefeller University.
Financial support was provided by the National Institutes of Health grants DK043806 and DK099443. The study was also supported by multiple core facilities including the Penn Diabetes Center (DK19525) Functional Genomics Core and Mouse Metabolic Phenotyping Core, Rockefeller University Proteomics Center, Princeton/Penn Regional Metabolomics Core, Vanderbilt MMPC (DK59637) and the Baylor Diabetes Center (DK079638) Mouse Metabolism Core.