Narrowing down genes involved in male fertility
A couple’s fertility depends on both the female’s and male’s ability to reproduce, which relies on thousands of genes working properly. Around the world, infertility affects about 15 percent of couples and approximately 10 percent of men who are attempting to sire offspring.

“Infertility is a serious condition and many of the genes responsible for it in men remain unknown,” said Dr. Martin M. Matzuk, Stuart A. Wallace chair, Robert L. Moody, Sr. chair, professor of pathology & immunology and director of the Center for Drug Discovery at Baylor.
The mouse is an animal model in which male infertility has been studied in detail up to the genetic level. In the male mouse, more than 1,000 genes are predominantly expressed in the testis, but their particular functions in reproduction are still a mystery.
In a report published June 12 in the Proceedings of the New York Academy of Sciences, researchers from Baylor College of Medicine, Osaka University, University of Oulu and the Wellcome Trust Sanger Institute have discovered that 54 of the mouse testis-enriched genes that also are expressed in humans are not necessary for male fertility.
Knowing which genes are required for male fertility is the first step toward better understanding the genetic changes that lead to male infertility and to design strategies to overcome them. At the same time, knowing which genes affect fertility would help design male contraceptive drugs, which is of increasing interest given the projections of the world’s population reaching 9 billion by 2050.
Women have been able to control their ability to reproduce with ‘the pill’ for over 50 years, but “there is still no contraceptive pill for men. It is therefore important to know all of the genes that cause infertility and also have knowledge of all of the proteins that could be testis-specific targets for a male pill. Our new study in mice would suggest that these 54 testis-enriched genes and their corresponding proteins would not play major roles in fertility in men and would be poor targets for a pill,” said Matzuk.
Knowing which genes are not involved in male fertility is important, the researchers say, because it allows scientists not to invest further financial, laboratory and human resources researching these genes. “I believe that researchers should concentrate on the essential genes first to get the big picture before spending grant funds and devoting significant efforts,” said corresponding author Dr. Masahito Ikawa, professor at the Research Institute for Microbial Diseases at Osaka University, Japan. This work helps narrow down the number of possible genes involved in male fertility.
Studying fertility genes in animal models is now faster and easier
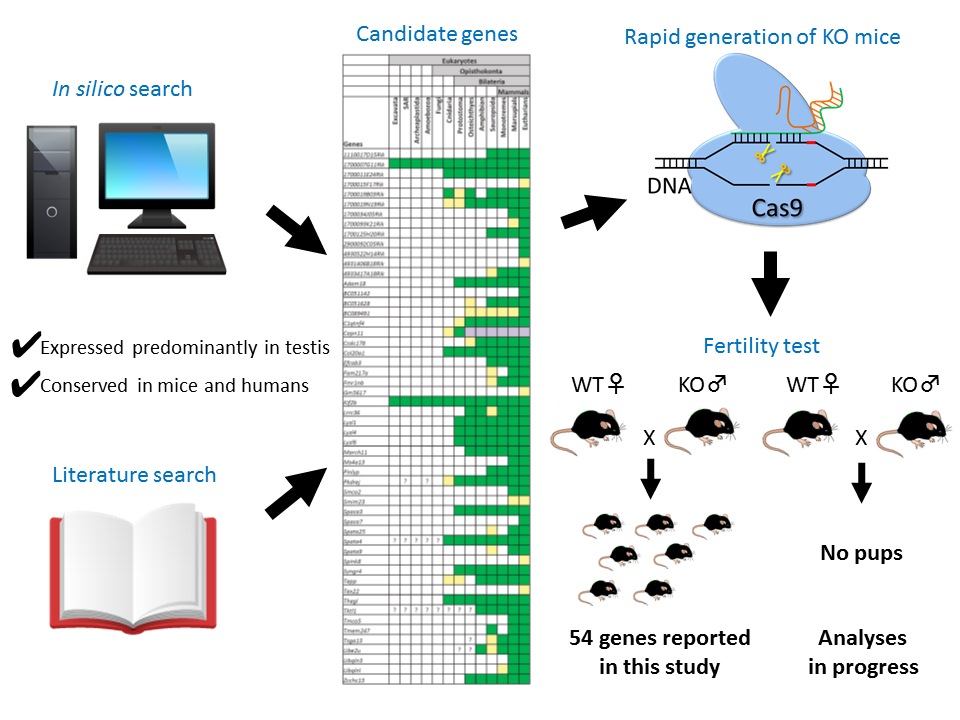
The study of fertility genes in animal models has been revolutionized by the development of techniques to create gene knockout mice. These mice have been modified so as not to express a particular gene, and the effect of lacking the gene on the animals’ fertility can be determined by examining the ability of breeding pairs to bear young. If a gene knockout mouse always sires pups, then the gene is considered to be not required for male fertility.
Until now, it has been difficult to create these knockout mouse models, but, recently, a new technique has been developed, called CRISPR/Cas9, which is boosting the studies of fertility genes. “Our laboratory introduced the CRISPR/Cas9 system that enables us to generate knockout mice in an easy, rapid and cost-effective way. By taking advantage of this robust technology, we generated 31 knockout mouse lines and analyzed their fertility within a short period of time,” said Ikawa.
“Our groups have shown that a large number of mammalian genes can be analyzed within a few years using the CRISPR/Cas9 system,” said co-first author Dr. Julio Castañeda, a postdoctoral fellow in Matzuk’s lab.
In addition, “Discovering essential functions of the genes of genetically modified animals is more beneficial than solely focusing on in vitro assays,” said co-first author Dr. Yoshitaka Fujihara, assistant professor at the Research Institute for Microbial Diseases at Osaka University.
The researchers began their collaborative studies with the Matzuk lab searching in silico databases for genes that are predominantly expressed in mouse testes and present in both mice and humans. Then, the labs in the team created knockout mice for individual genes to determine their effect on the animals’ fertility.
“Our results indicate that mutations in these 54 genes are not a major cause of male infertility,” said co-first author Dr. Haruhiko Miyata, assistant professor at the Research Institute for Microbial Diseases at Osaka University.
The study also suggests the possibility of redundancy in the functions of proteins from the testis. Redundancy would allow the animal to remain fertile after one gene fails because another gene would replace its function. Further studies would be required to explore this possibility.
###
Other contributors to this work include Zhifeng Yu, Denise Archambeault, Ayako Isotani, Daiji Kiyozumi, Maya Kriseman, Daisuke Mashiko, Takafumi Matsumura, Ryan Matzuk, Masashi Mori, Taichi Noda, Asami Oji, Masaru Okabe, Renata Prunskaite-Hyyrylainen, Ramiro Ramírez-Solís, Yuhkoh Satouh and Qian Zhang.
This work was supported by MEXT grants (26830056, 15H05573, 15K14366, 15K14367, 15K06999, 15K18387, 15J04519, 25112007, 25250014 and T15K21737a), the Takeda Science Foundation grant, Wellcome Trust grants (079643 and 098051), National Institutes of Health grants (U01-HD060496, U01-HG004080, and 5T32HD007165-35), Osaka University International Joint Research Promotion Program, the Academy of Finland and the Sigrid Juselius Foundation.