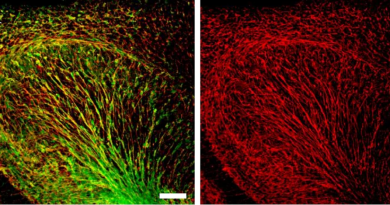
From bench to bedside: Osteoarthritis gene therapy developed in the lab at Baylor is in clinical trials nationwide
Developing novel therapies for disease is a slow process that can take decades of trial and error in the lab before the therapy can be evaluated for safety and efficacy in human patients. The lab of Dr. Brendan Lee, professor and chair of the Department of Molecular and Human Genetics and Robert and Janice McNair Endowed Chair at Baylor College of Medicine, has worked for years on development of an adenovirus gene therapy for osteoarthritis. Today, that therapy is showing promising results in Phase 1 clinical trials.
Starting with adenovirus
Baylor’s work on gene therapy for genetic diseases started in the 1990s in the labs of Dr. Arthur Beaudet and Dr. C. Thomas Caskey, using adenoviruses to treat inborn errors of metabolism.

“The adenovirus is probably the most effective way to deliver DNA into a cell,” Lee said.
“The problem is that the early versions of these vectors were quite immunogenic and caused systemic inflammation. The virus stimulated such a strong immune response that it could harm the patient when delivered directly into the blood for gene therapy. Clearly, we knew major advancements were needed to apply them to chronic diseases.”

A major advancement in field came in the early 2000s with the development of the helper-dependent adenovirus, also known as high-capacity adenovirus. Researchers at Baylor and elsewhere engineered the high-capacity adenovirus by removing the viral genome, preventing the virus from producing viral proteins that trigger the immune response and thereby making the therapy much safer for patients. Experiments at Baylor led by Dr. Philip Ng showed that the high-capacity adenovirus would continue to express genetically modified DNA for seven years without triggering a severe immune response.
Targeting osteoarthritis
As advances in high-capacity adenovirus gene therapy continued over the next decade, Lee’s lab members decided to combine their work on gene therapy with their work on skeletal disorders like osteoarthritis.
The Centers for Disease Control and Prevention estimates that 33 million adults in the U.S. have osteoarthritis, but there is no cure.
Drug development has been difficult because osteoarthritis disease progression is slow and potential therapies must be safe for patients to take over a long period of time,” Lee said.
Using high-capacity adenovirus gene therapy in the knee joint provided an advantage because the joint is a closed system. Injections to the knee joint do not enter the bloodstream and, therefore, are protected from the body’s immune system.

Lee’s lab focused on gene therapy with interleukin-1 receptor antagonist (IL-1Ra), an anti-inflammatory protein that had been proven by other labs to be an effective target for slowing osteoarthritis in many animal models, although the effects in human trials with the recombinant protein and antibodies against the IL-1 pathway have been mixed. Instead of overexpressing this protein to block all inflammation, the lab developed a unique approach. Led by Dr. Kilian Guse, a postdoctoral associate in the Lee lab at the time, they created a high-capacity adenovirus vector with an NFkB responsive promoter to turn on IL-1Ra expression only when there is inflammation in the joint.
“Inflammation can be both good and bad,” Lee said. “Tissue regeneration to heal a wound requires some inflammation. But too much inflammation can lead to autoimmune disease or tissue damage in the context of arthritis.”
Preclinical testing of the therapy showed promising results in mice and horse models. The therapy was licensed to Guse’s startup company, then called GeneQuine and now known as GQ Bio Therapeutics.
“The aim of this program is to develop a drug for osteoarthritis with sustained symptomatic and disease-modifying efficacy, and we believe that a gene therapy approach has the highest potential to achieve this,” said Guse, CEO and co-founder of GQ Bio Therapeutics.
High-capacity adenoviral vectors are ideally suited for osteoarthritis gene therapy as they very efficiently deliver their DNA payload to joint cells, much more efficiently than the commonly used AAV vectors of which significantly higher doses are needed.”
Moving to clinical trial
The therapy was next acquired by Flexion Therapeutics, Inc., where it eventually moved to a Phase 1 clinical trial. In 2021, Pacira BioSciences, Inc. acquired Flexion Therapeutics and the osteoarthritis gene therapy, now called PCRX-201.
In March, the therapy became the first-ever gene therapy candidate to receive Regenerative Medicine Advanced Therapy (RMAT) designation from the U.S. Food and Drug Administration, a designation that helps guide efficient drug development and supports accelerated approval.
This month, Pacira presented two-year safety and efficacy data from the Phase 1 trial. Results showed that treatment with PCRX-201 for moderate to severe osteoarthritis of the knee provided sustained improvements in knee pain, stiffness and function to 104 weeks following local administration, indicating a potential for sustained clinical efficacy. Read more of the trial results here. Pacira is planning a Phase 2, double-blind, active-controlled study planned for next year.
“With 72 knee osteoarthritis patients in the PCRX-201 Phase 1 clinical trial, it has by far been the largest clinical trial with a high-capacity adenovirus and in the field of osteoarthritis gene therapy in general,” Guse said. “We are excited to work together with Pacira on the further development of PCRX-201 toward market approval.”
This therapy has the potential to be the first disease-modifying therapy for osteoarthritis, for which there is a huge unmet need,” Lee said. “These promising clinical trial results are a big step forward in a years-long development journey that started in the lab at Baylor.”
By Molly Chiu
Follow From the Labs on X @BCMFromtheLabs and Instagram!