The multi-tasking nature of the hormone asprosin
When Dr. Atul Chopra, a medical geneticist at Baylor College of Medicine, and his colleagues discovered in 2016 a new hormone called asprosin that regulates blood-glucose levels, the possibilities opened for future treatments that could potentially benefit millions of people living with type 2 diabetes. Asprosin’s reach, however, goes beyond promoting hepatic glucose production. A 2017 study by Chopra and his colleagues shows that the hormone also can cross the blood-brain barrier and stimulate the hunger center in the hypothalamus to control appetite and body weight.
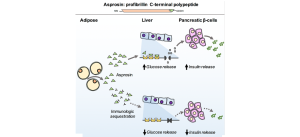

“We discovered asprosin when studying individuals affected by a rare medical condition called neonatal progeroid syndrome,” said Chopra, Caroline Wiess Law Scholar and assistant professor of molecular and human genetics and molecular and cellular biology at Baylor College of Medicine. “We found that patients with neonatal progeroid syndrome have a mutation in the FBN1 gene that causes them to lack a small piece of the fibrillin-1 protein. In individuals without the FBN1mutation, this small piece, which we named asprosin, is cut and released into the circulation from the end of the protein.”
One of the cardinal features that defines neonatal progeroid syndrome is extreme thinness. To understand the cause of this very low body weight, Chopra and his colleagues assessed the food intake pattern and metabolic rate of the patients.
“We evaluated the patients’ energy balance in a chamber called an indirect calorimeter,” said Chopra, who also is a member of the Dan L Duncan Comprehensive Cancer Center at Baylor. “This allowed us to measure how much food they ate relative to the number of calories they burned every day. This test gave us a clear answer.”
“Compared with individuals with normal weight, neonatal progeroid syndrome patients have abnormally low appetite. Because these patients have low blood asprosin levels due to their mutations, we wondered whether asprosin was in fact necessary to maintain normal appetite in people.”
What does asprosin do?
To investigate how the mutation affected the patients’ appetite, the researchers genetically engineered mice to carry the same genetic mutation the patients have. The result was mice that mimicked the human condition; they had low blood asprosin levels, low appetite and were very thin.
“In this mouse model we were able to reverse the low appetite simply by administering asprosin to the mice,” Chopra said.
To understand how asprosin controls appetite, the researchers turned to colleagues at the USDA/ARS Children’s Nutrition Research Center who specialize in studying brain circuits that control appetite.
“In collaboration with Dr. Yong Xu’s laboratory, we found that in the brain asprosin interacts with neurons in the appetite center of the hypothalamus,” Chopra said. “There are two types of neurons involved in appetite control. One type, the AgRP neurons, stimulates appetite while the other type, POMC neurons, suppresses it. Asprosin works on both types of neurons in an opposite manner; it activates appetite-stimulating AgRP neurons and it deactivates appetite-suppressing POMC neurons.”

“The effects of asprosin on AgRP and POMC neurons appear to be quite unique, as we did not find asprosin changing the firing activities of other appetite-regulating neurons,” said Xu, associate professor of pediatrics and nutrition and of molecular and cellular biology at Baylor and a corresponding author of this work.
The resulting effect of these two asprosin actions in the brain is an increase in appetite, a phenomenon that is deficient in individuals and mice with neonatal progeroid syndrome.
“Although we know some intracellular signals, for example cAMP, are required to mediate asprosin’s appetite-stimulating effects on AgRP neurons, the receptors for the hormone remain to be identified,” Xu said. “This is our current focus.”
Asprosin and obesity
In addition to studying individuals with neonatal progeroid syndrome who have low levels of asprosin, the researchers also studied individuals with obesity and found that they had increased levels of blood asprosin.
“Although we don’t yet understand the mechanism behind this increase, it gives us a possible opportunity to treat obesity by regulating blood asprosin levels,” Chopra said. “We developed an antibody against asprosin that can neutralize asprosin function completely. We administered this antibody to obese mice and found that the mice ate less and lost weight. More work remains to be done; however, these results potentially open the doors on a completely new way to treat obesity.”
In their previous work, the researchers had found that treating obese mice with anti-asprosin antibodies also reduced their levels of blood glucose, which suggests that asprosin immunotherapy can potentially also be used to treat diabetes.
Read the complete study in the journal Nature Medicine.
Visit the Chopra lab website and read his interview on CBS Evening News with Scott Pelley.

Other contributors to this work include Clemens Duerrschmid, Yanlin He, Chunmei Wang, Chia Li, Juan Bournat, Chase Romere, Pradip K. Saha, Mark Lee, Kevin J. Phillips, Mahim Jain, Peilin Jia, Zhongming Zhao, Monica Farias, Qi Wu, Dianna M. Milewicz, V. Reid Sutton, David D. Moore, Nancy F. Butte and Michael J. Krashes. The researchers are affiliated with one of more of the following institutions, Baylor College of Medicine, the USDA/ARS Children’s Nutrition Research Center, the National Institutes of Health and the University of Texas Health Science Center at Houston.
This work was supported in part by the Baylor College of Medicine Mouse Metabolic Core funded by the National Institutes of Health (P30 DK079638), the Monoclonal Antibody/Recombinant Protein Expression Shared Resource funded by the NIH (P30 CA125123), the Mouse Phenotyping Core, funded by the NIH (UM1HG006348), and the University of North Carolina at Chapel Hill Protein Expression Purification Core, funded by the NIH (P30 CA016086). Further support was provided by American Heart Association postdoctoral fellowships (16POST29630010 and 16POST27260254), the American Diabetes Association (1-17-PDF-138), the Intramural Research Program of the NIH, the NIDDK (DK075087, 1K08DK102529 and DK101379), the NLM (LM012806), the USDA/CRIS (3092-5-001-059), the Chao Physician-Scientist Award, the Caroline Wiess Law Scholar Award and a departmental laboratory startup package.




awesome blog, great content about asprosin.. keep posting.. regards from Indonesia