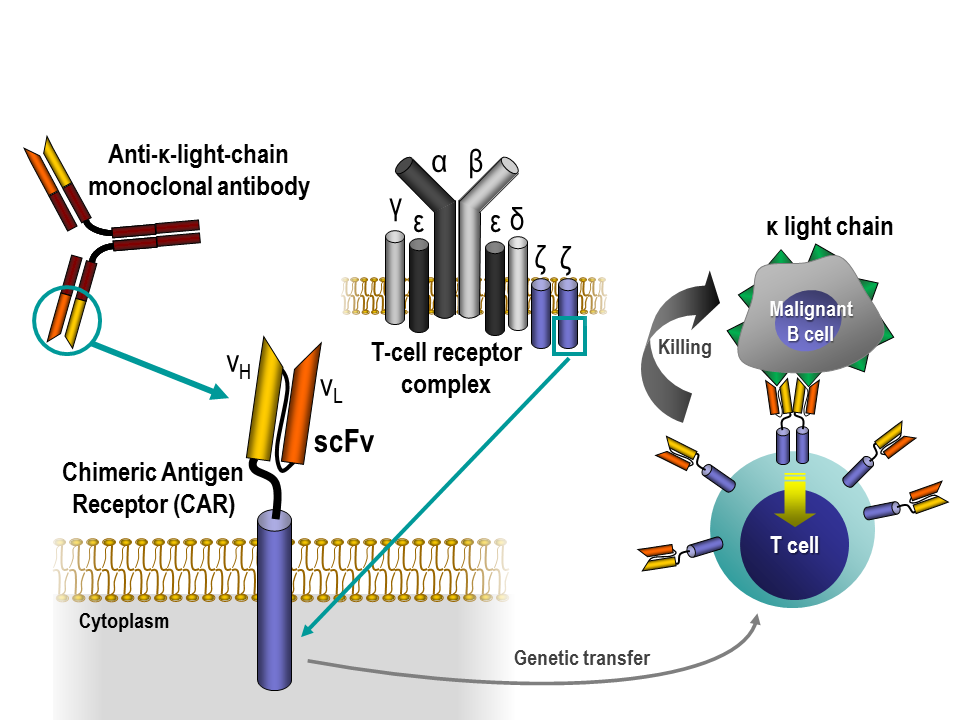
Targeting B-cell malignancies with κ-specific T cells can lead to complete clinical responses
Treating cancer with the patient’s own immune cells has been successful. In particular, treating B cell malignancies with the patients’ own immune T cells modified so that they target specific B cell markers present on malignant B cells. However, if T cells remain present in the body for prolonged periods of time, which might be necessary for clinical success, the total number B cells, an important branch of the immune response, would be substantially reduced.

To solve this conundrum, Dr. Carlos A. Ramos, associate professor in the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston Methodist Hospital and Texas Children’s Hospital, and his colleagues from Baylor have designed and tested a variation of the above treatment. They armed T cells with the means to specifically target a subpopulation of B cells that encompasses the malignant ones, instead of all malignant and healthy B cells. The promising results of their phase I clinical trial appear in the Journal of Clinical Investigation.
B cells can be members of two mutually exclusive populations, each having either a κ- or a λ-light chain on their surface. B cell malignancies, such as non-Hodgkin lymphoma, chronic lymphocytic leukemia or multiple myeloma, express on their surface either κ- or λ-light chains, but not both.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said Ramos.
The researchers isolated T cells, members of the immune system that identify and destroy abnormal cells, from patients with B cell malignancies expressing the κ-light chain on their surface. Then, they modified each patients’ T cells so they could target the κ-light chain on the surface of malignant B cells. The modified T cells were infused back into the patient, and each patient monitored for progression of the disease and side effects.
The main goal of phase I clinical trials is to determine the safety of a new medical treatment in human patients. “We found the treatment to be feasible and safe at all the dose levels studied,” said Ramos. “In two of the nine patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia, our treatment resulted in complete remission, and a third patient had a partial response. Four of the seven patients with multiple myeloma had a stable disease lasting two to 17 months after the treatment.”
This new approach to treating B cell malignancies can potentially be less toxic than traditional treatments and have temporary side effects, as well as preserve a part of the B cell responses; that is, the body’s ability to produce normal, λ-type antibodies.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” said Ramos.
###
Others who contributed to this work include Gianpietro Dotti (corresponding author), Barbara Savoldo, Vicky Torrano, Brandon Ballard, Huimin Zhang, Olga Dakhova, Enli Liu, George Carrum, Rammurti T. Kamble, Adrian P. Gee, Zhuyong Mei, Bambi Grilley, Cliona M. Rooney, Malcolm K. Brenner, Helen E. Heslop and Meng-Fen Wu, all of Baylor.
Support for the research has come from the National Institutes of Health, the National Cancer Institute (grants 3P50CA126752 and 5P30CA125123) and the Leukemia and Lymphoma Society Specialized Center of Research (grant 7018). The clinical trial also received support from The Institute for Clinical and Translational Research at Baylor College of Medicine and shared resources of the Dan L Duncan Comprehensive Cancer Center support grant 5P30CA125123, and the National Gene Vector Biorepository.
This clinical trial was registered under ClinicalTrials.gov NCT00881920.
While this clinical trial was being conducted, the Center for Cell and Gene Therapy at BCM had a Collaborative Research Agreement with Celgene Corporation and Bluebird Bio.